Water-soluble polymer derivative of Venotog
A technology of water-soluble polymers and drugs, which is applied in the field of drugs for the treatment or alleviation of cancer, can solve the problems of unapproved, increased toxicity, and inability to improve the therapeutic effect of irinotecan, and achieve excellent balance and reduce the burden.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
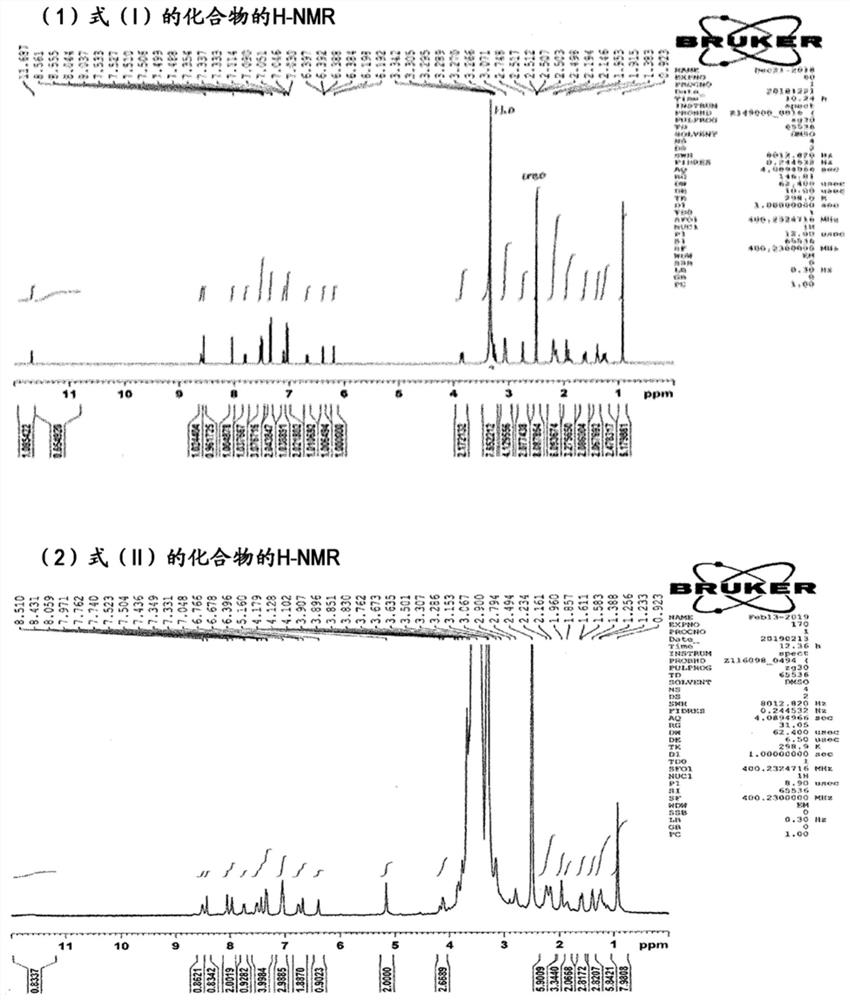
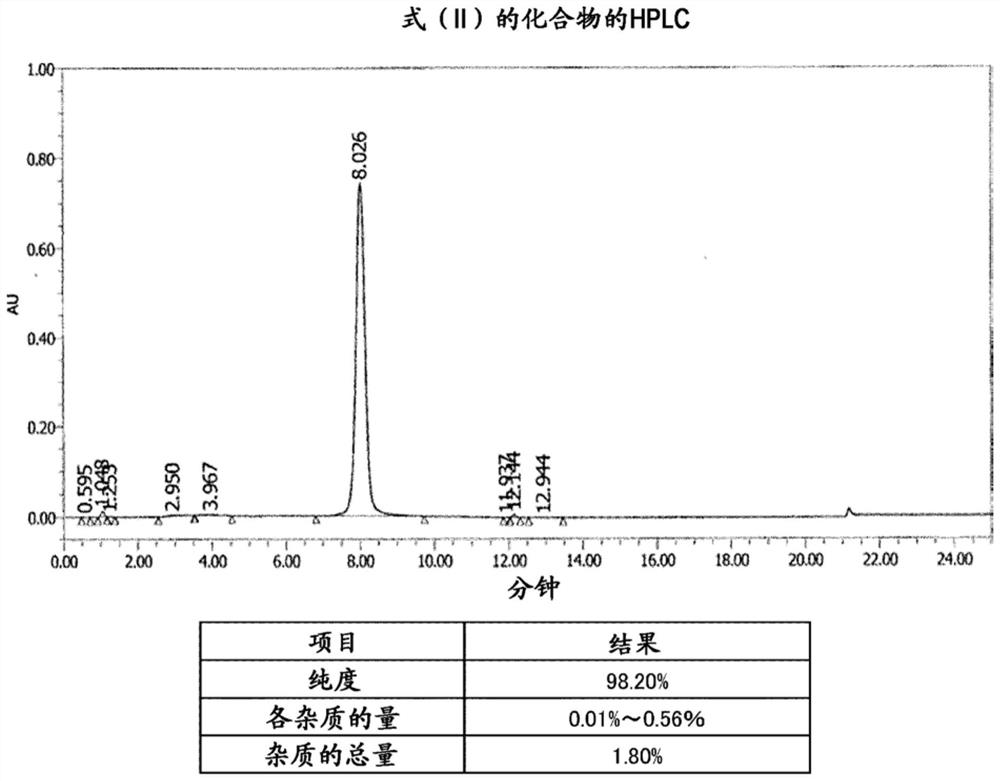
[0088] [Example 1] Synthesis and analysis of compounds of formula (II)
[0089] Under a nitrogen atmosphere, CTPEG (4.530 g, 1 equivalent) was added to 55 mL of a DMF solvent, and heated at 50° C. to dissolve uniformly. Add N,N-diisopropylethylamine (hereinafter referred to as "DIC") (0.296 g, 20 equivalents), 1-hydroxybenzotriazole (hereinafter referred to as "HOBT") (0.258 g , 6 equivalents) and venetoclax (0.474 g, 4.8 equivalents). After continuing to stir at 60°C for 6 hours, cool to 40°C, then dropwise add methyl tetrabutyl ether (hereinafter referred to as "MTBE") heated to 30°C over 20 minutes while stirring, and then cool over 60 minutes , after stirring for 30 minutes, the resulting crystals were filtered and collected, washed with 20 mL of MTBE, and the obtained crystals were dissolved in 20 mL of absolute ethanol heated to 40° C., and 70 mL of MTBE was added dropwise over 20 minutes, and after 60 Minutes cooled to 0 ° C, stirred for 30 minutes, the resulting prec...
Embodiment 2
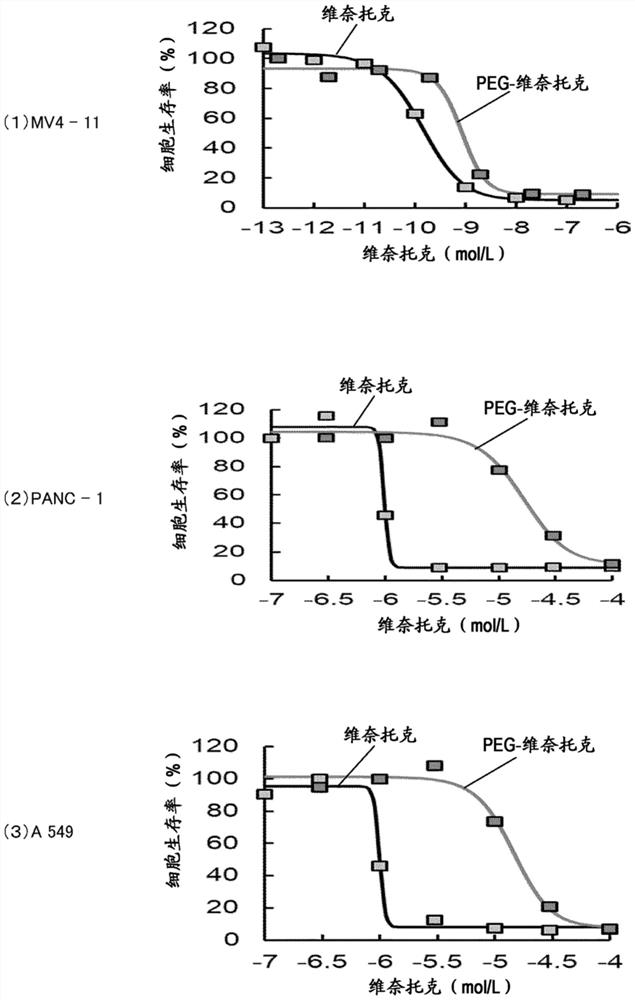
[0090] [Example 2] Cell killing effect of the compound of formula (I) and the compound of formula (II)
[0091] The concentration dependence of the cell killing effect of the compound of formula (I) and the compound of formula (II) was investigated in the following manner.
[0092] Using MV4-11 of the human acute myeloid leukemia cell line, study the respective IC of the compound of formula (I) and the compound of formula (II) 50 (50% inhibition rate) (see image 3 (1)). As a result, the IC of the compound of formula (I) 50 (50% inhibition rate) is 0.16pM, the IC of the compound of formula (II) 50 It was 0.85 pM, and both compounds were confirmed to have high inhibitory rates. Among them, the protein binding rate of the compound of formula (I) is high (≥99.9%), so a medium with low serum content (Opti-MEM) is used.
[0093] Using PANC-1 of a human pancreatic cancer cell line, the ICs of the compound of formula (I) and the compound of formula (II) were studied 50 (50% inh...
Embodiment 3
[0095] [Example 3] Cell killing effect of compound of formula (II) and protease activity of cancer cells
[0096] The protease activity measurement results of U937 (human histiocytic lymphoma cell line), MV4-11 (human acute myeloid leukemia cell line), PANC-1 (human pancreatic cancer cell line) and A549 (human lung cancer cell line) are shown At Figure 4 . Protease activity using AAT Bioquest's "Amplite TM Universal Fluorimetric Protease Activity Assay Kit*Green Fluorescence*” was measured, and the fluorescent intensity obtained was corrected by the amount of protein measured by Bio-Rad’s “DC ProteinAssay”. As a result, the formula measured in the above-mentioned embodiment 2 ( II) The compound confirms the correlation between the 50% inhibitory concentration of MV4-11, PANC-1 and A549, and the protease activity of cancer cell lines. That is, it is confirmed that the protease activity of cancer cells is higher, based on the formula (II ) the higher the inhibition rate of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com