Application of dihydroergomine in preparation of antitumor drugs
A technology of dihydroergotamine and anti-tumor drugs, which is applied in the direction of anti-tumor drugs, drug combinations, active ingredients of heterocyclic compounds, etc., and can solve problems such as the lack of anti-tumor therapeutic effects of dihydroergotamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
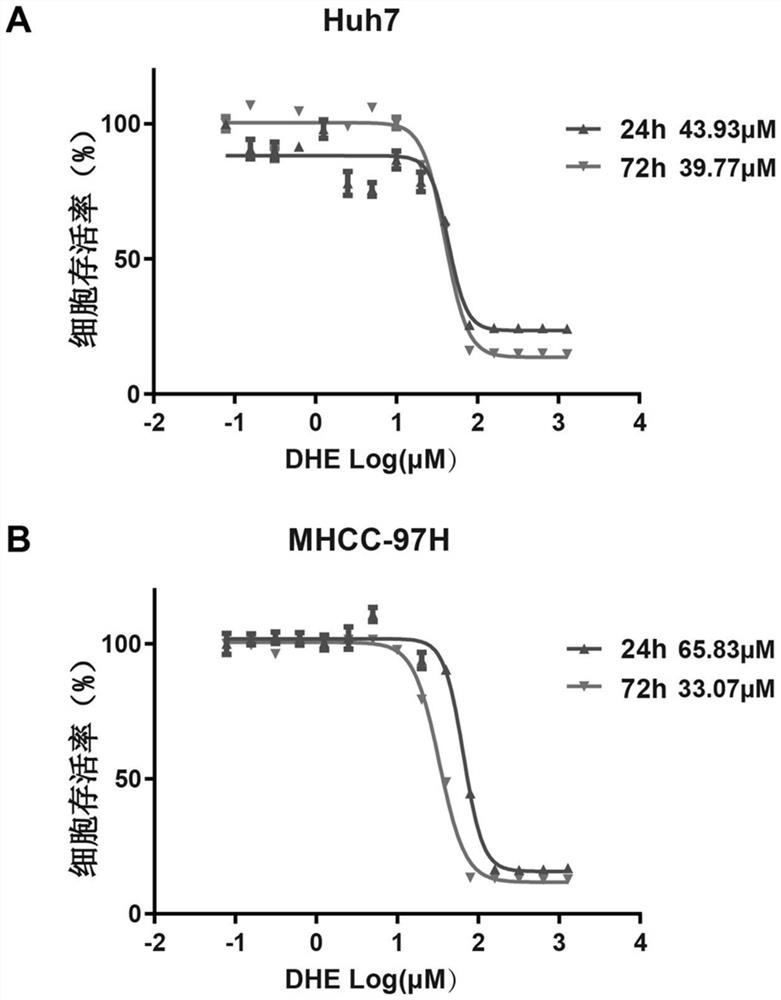
[0044] 1. Cell culture and plating: the high-glucose DMEM medium containing 10% fetal bovine serum is used to cultivate Huh7 and MHCC-97H liver cancer cells, and the Huh7 and MHCC-97H liver cancer cells in the logarithmic growth phase are inoculated at 6000 cells / well. in a 96-well plate.
[0045] 2. Drug treatment: After the liver cancer cells adhere to the wall, dihydroergotamine drug is diluted to treat the cells, so that the final concentration of dihydroergotamine reaches 0.078125μM, 0.15625μM, 0.3125μM, 0.625μM, 1.25μM, 2.5μM, 5μM , 10 μM, 20 μM, 40 μM, 80 μM, 160 μM, 320 μM, 640 μM and 1280 μM, set 3 duplicate wells for each group, the final volume of medium in each well was 100 μL, let the drug act on the cells for 24 hours and 72 hours.
[0046] 3. Detection of cell survival rate: Prepare CCK-8 working solution according to the ratio of CCK-8 reagent (Dongren Chemical Technology): complete medium = 1:10, discard the cell culture medium in each well, and add 100 μL CCK...
Embodiment 2
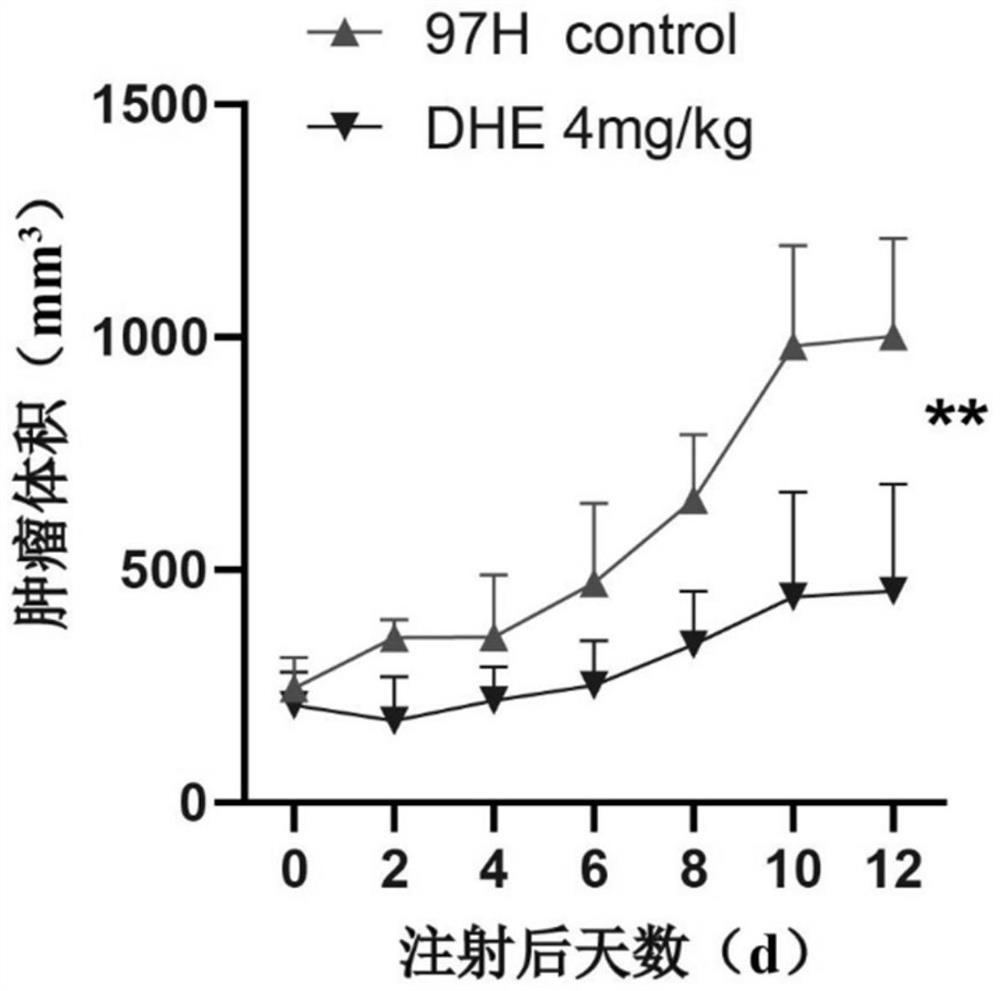
[0048] 1. Construct a nude mouse subcutaneous tumor model: culture human liver cancer cells (MHCC-97H) in vitro, digest the cells in the logarithmic growth phase with trypsin, centrifuge at 800rpm×3min, wash the liver cancer cells with PBS reagent 3 times, and adjust the number of cells after counting 1×10 8 / mL, inject 100 μL of liver cancer cell suspension into the back of BALB / c nude mice (4-6 weeks old, male or female) for subsequent experiments.
[0049] 2. Grouping: the tumor of the tumor-bearing mice is about 200mm long 3 , measure the body weight and tumor volume of the mice one by one, eliminate outliers in body weight and tumor volume, and then divide the tumor-bearing mice into a control group and an experimental group randomly.
[0050] 3. Treatment: According to the weight of the mice, the experimental group (DHE / DHE 4mg / kg) was given intraperitoneal injection of 5 μL / g dihydroergot injection every day; the control group (control / 97H control) was given daily intr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com