Binding proteins specific for ha-1h and uses thereof
A protein-binding and specific technology, applied in peptide/protein components, animal/human proteins, immunoglobulin superfamily, etc., can solve the problems of lack of in vivo persistence of T cells and in vivo anti-leukemia reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
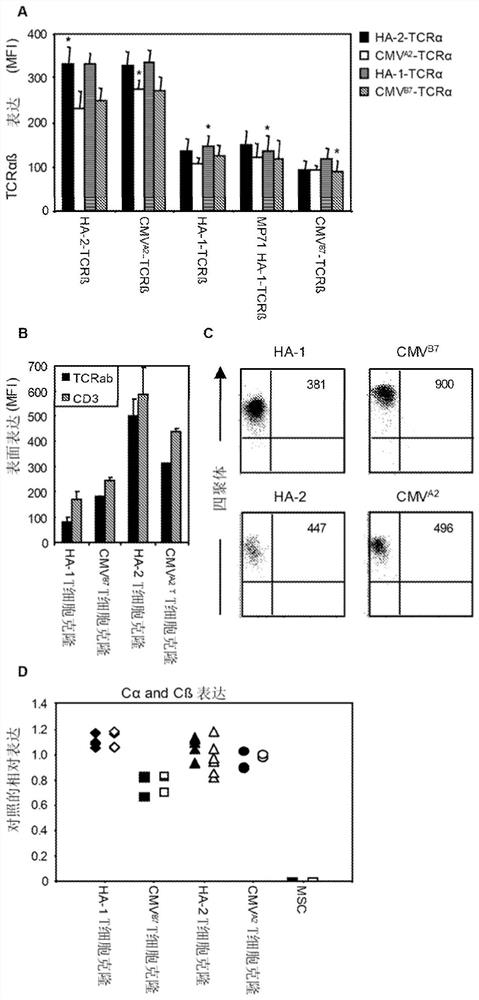
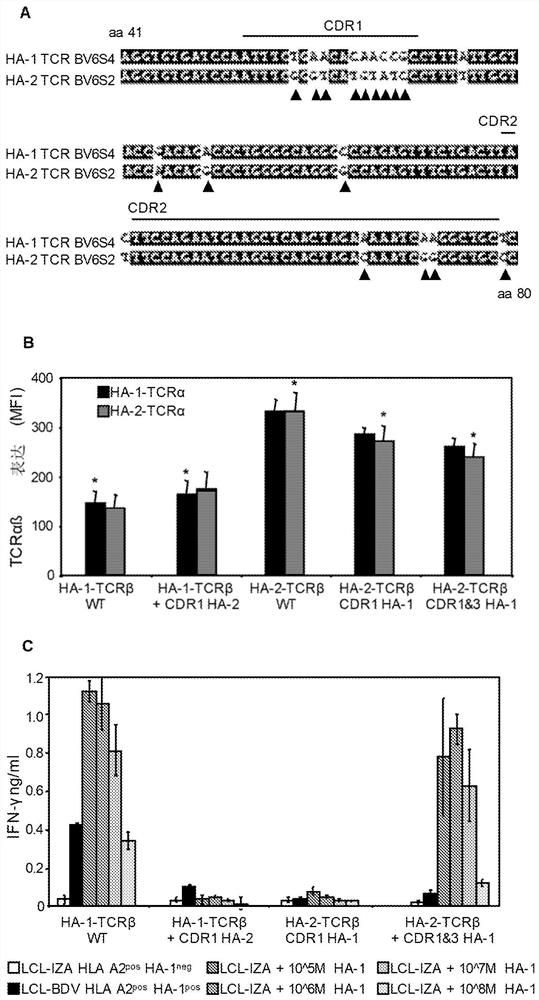
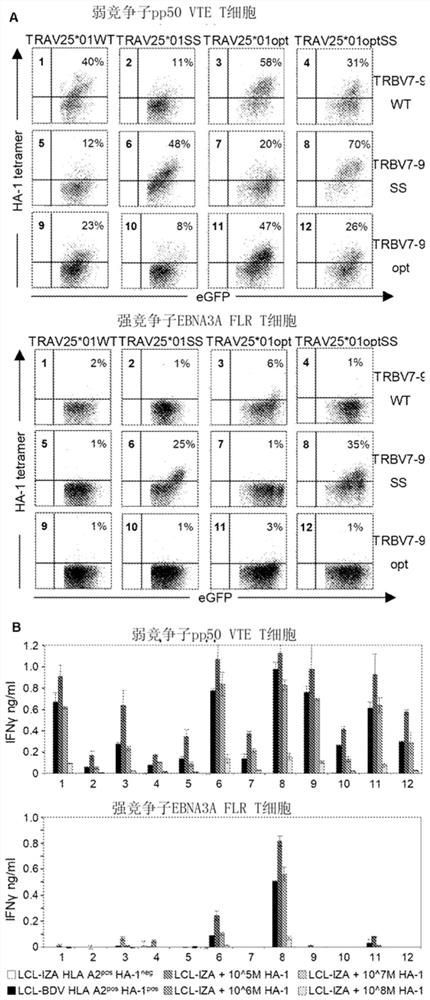
[0443] TCR gene transfer is an attractive strategy to modify T cells with well-defined specificities in a short period of time. Recently, the effectiveness of TCR transfer was demonstrated in melanoma or synoviocyte sarcoma patients treated with TCR-modified autologous T cells. In order to engineer T cells capable of selective GvL without GvHD, we prefer to transfer HA-1-TCR. To expand the applicability of acquired T cell therapy in hematological malignancies, we initiated a clinical study using HA-1-TCR-transferred virus-specific T cells. We sequenced the TCR chains of three HA-1-specific T cell clones, M2, M7 and FK47.83 (Table 4).
[0444]
[0445]
[0446] Table 4. HA-1-TCR sequences of clones M2, M7 and FK47.83.
[0447] As previously described, we again observed that all three HA-1-specific T cell clones expressed a β chain with a similar V region (TRBV7-9) (4). A LZRS retroviral construct encoding the TCR alpha and beta chains of M2 and M7 was prepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com