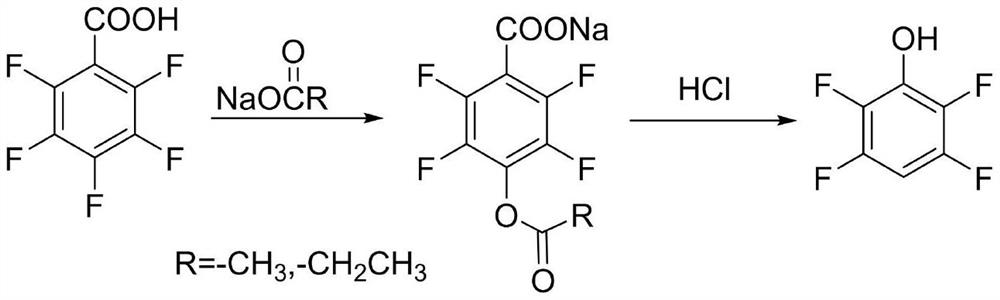
A kind of method of synthesizing 2,3,5,6-tetrafluorophenol
A technology of tetrafluorophenol and pentafluorobenzoic acid, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as complicated operation, high cost, increased energy consumption, etc., and achieve convenient operation and environmental pollution. small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 404 grams of DMF and 202.3 grams of sodium acetate were placed in a 1 L glass reactor. Add 106.0 g of pentafluorobenzoic acid at a temperature of 50-55 °C. The temperature was raised and refluxed for 6 hours. HPLC detects that the raw material content of the reaction solution is lower than 1.0% and drops below 80°C, and 350.0 grams of 30% concentrated hydrochloric acid is added to the kettle to adjust the pH to less than 1. Then, the temperature was raised and refluxed for 5 hours. After refluxing, steam distillation was performed to separate the lower organic matter. The lower organic matter was rectified to obtain 74.0 g of 2,3,5,6-tetrafluorophenol, which turned into white crystals after cooling down, the yield was 89%, and the HPLC purity was 99.9%.
Embodiment 2
[0026] 305 grams of DMSO and 202.3 grams of sodium acetate were placed in a 1 L glass reactor. Add 106.0 g of pentafluorobenzoic acid at a temperature of 50-55 °C. The temperature was raised and refluxed for 2 hours. HPLC detects that the raw material content of the reaction solution is lower than 1.0% and drops below 80°C, and 350.0 grams of 30% concentrated hydrochloric acid is added to the kettle to adjust the pH to less than 1. Then, the temperature was raised and refluxed for 5 hours. After refluxing, steam distillation was performed to separate the lower organic matter. The lower organic matter was rectified to obtain 76.6 g of 2,3,5,6-tetrafluorophenol, which turned into white crystals after cooling down, the yield was 92%, and the HPLC purity was 99.8%.
Embodiment 3
[0028] 800 grams of water and 202.3 grams of sodium acetate were put into a 1 L glass reactor. Add 106.0 g of pentafluorobenzoic acid at a temperature of 55-60 °C. The reaction was carried out under reflux for 40 hours. HPLC detects that the raw material content of the reaction solution is lower than 1.0% and drops below 80°C, and 350.0 grams of 30% concentrated hydrochloric acid is added to the kettle to adjust the pH to less than 1. Then, the temperature was raised and refluxed for 5 hours. After refluxing, steam distillation was performed to separate the lower organic matter. The lower organic matter was rectified to obtain 73.2 g of 2,3,5,6-tetrafluorophenol, which turned into white crystals after cooling down, the yield was 88%, and the HPLC purity was 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com