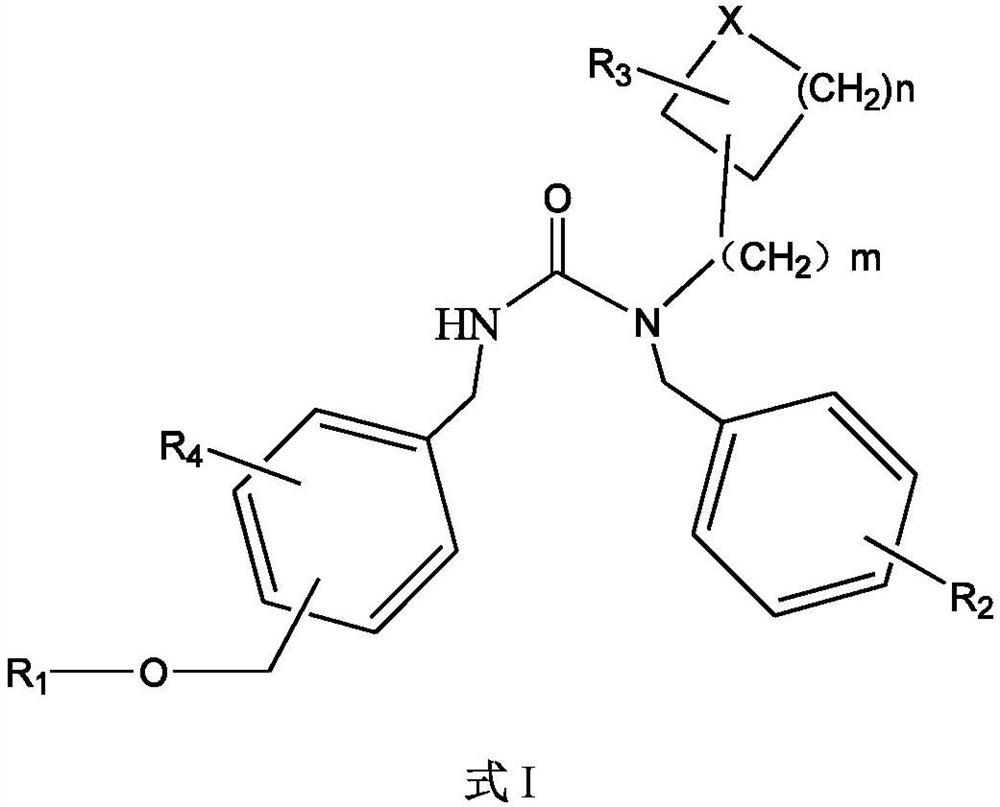
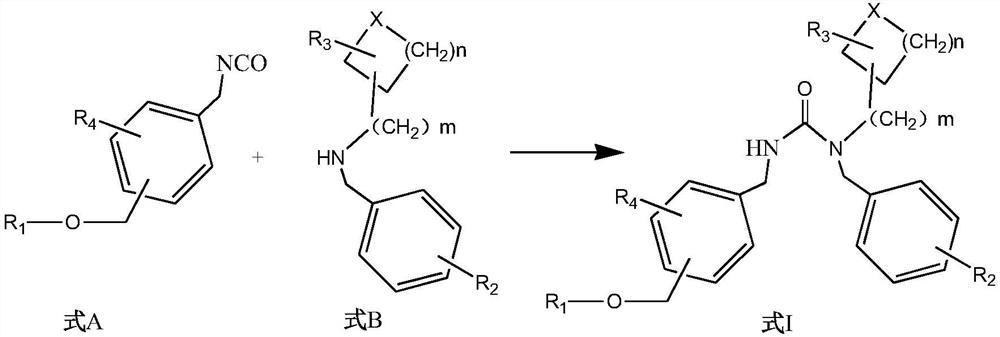
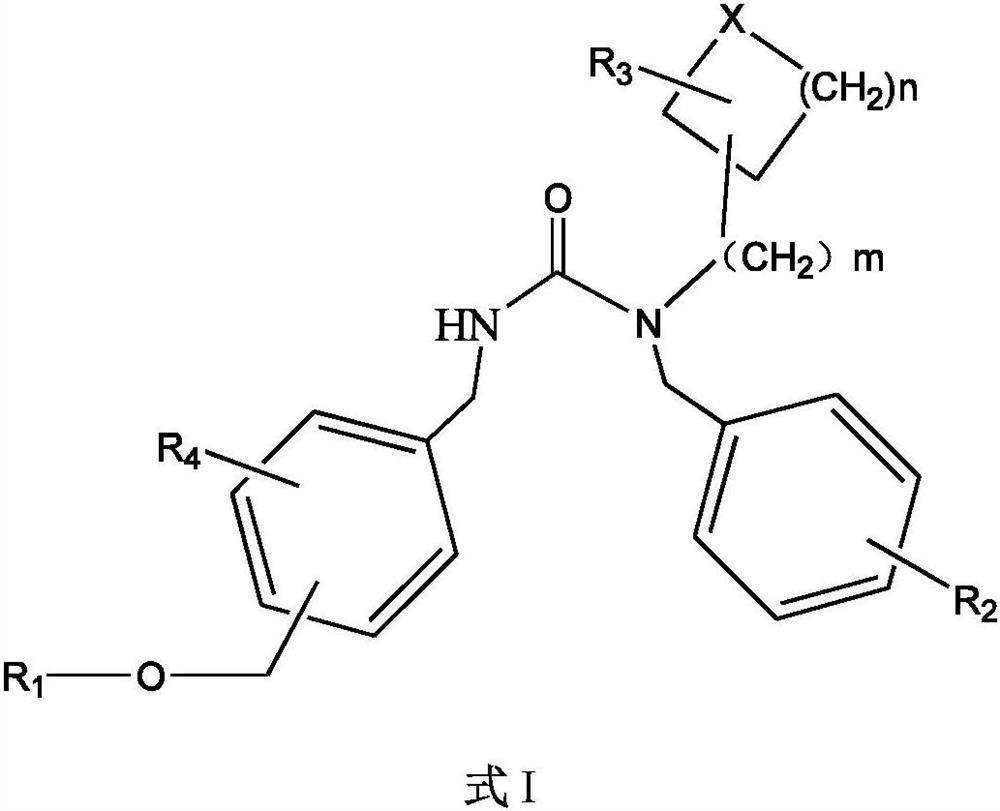
5HT2A receptor antagonist, and preparation and application thereof
A R1OCH2-, solvate technology, applied in the field of medicine, can solve problems such as abnormal motor function and type 2 diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Example 1: 3-(4-isopropoxymethylbenzyl)-1-(4-fluorobenzyl)-1-(1-methylpiperidin-4-yl)urea (III-1 ) Preparation (ER10067)
[0135]
[0136] To a solution of trimeric phosgene (49.7 mg, 0.167 mmol, 1.0 equiv.) in dichloromethane was added dropwise 4-isopropoxymethyl-1-benzylamine (I-1) (30 mg, 0.167 mmol , 1.0 equiv.) in tetrahydrofuran (1.0 mL). A solution of triethylamine (0.070 mL, 3.0 equiv.) in dichloromethane (2.0 mL) was then added dropwise. After precipitation, the residue was redissolved in dichloromethane (3.0 mL), and (4-fluoro-benzyl)-(1-methylpiperidin-4-yl)-amine (II-1) was added ( 39.6 mg, 1.0 equiv.) in tetrahydrofuran (2.0 mL). The mixture was stirred at room temperature for 2 hours. After precipitation, the crude product was purified by silica gel column to give the final product.
Embodiment 2
[0137] Example 2: 3-((4-isopropoxymethyl)benzyl)-1-(4-fluorobenzyl)-1-((1-methylpiperidin-4-yl)methyl ) Preparation of urea (III-2) (ER10235)
[0138]
[0139] 3-((4-isopropoxymethyl)benzyl)-1-(4-fluorobenzyl)-1-((1-methylpiperidin-4-yl)methyl)urea (III -2) is prepared analogously to 3-(4-isopropoxymethylbenzyl)-1-(4-fluorobenzyl)-1-(1-methylpiperidin-4-yl)urea ( III-1) Preparation: Using 4-isopropoxymethyl-1-benzylamine (I-1) (30 mg, 0.167 mmol, 1.0 equiv.) and N-(4-fluorobenzyl)- 1-(1-methylpiperidin-4-yl)methanamine (II-2) (39.6 mg, 0.167 mmol, 1.0 equiv.), silica gel column purification gave the final product (13 mg, yield 18%). LCMS: M+1] + 442.8.
Embodiment 3
[0140] Example 3: 3-((4-isopropoxymethyl)benzyl)-1-(4-fluorobenzyl)-1-(((R)-1-(1-methylpyrrolidine Preparation of -3-yl))methyl)urea (III-3) (ER10236)
[0141]
[0142] 3-((4-isopropoxymethyl)benzyl)-1-(4-fluorobenzyl)-1-(((R)-1-(1-methylpyrrolidin-3-yl )) methyl) urea (III-3) is prepared analogously to 3-(4-isopropoxymethylbenzyl)-1-(4-fluorobenzyl)-1-(1-methylpiper Preparation of pyridin-4-yl)urea (III-1): Using 4-isopropoxymethyl-1-benzylamine (I-1) (30 mg, 0.167 mmol, 1.0 equiv.) and (S) -N-(4-fluorobenzyl)-1-(1-methylpyrrolidin-3-yl)methanamine (II-3) (37.2 mg, 0.167 mmol, 1.0 equiv.), purification on silica gel column gave Final product (5.3 mg, yield 7.0%). LCMS: [M+1] + 428.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com