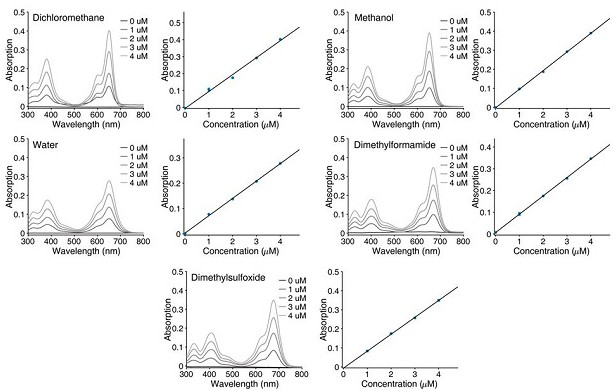
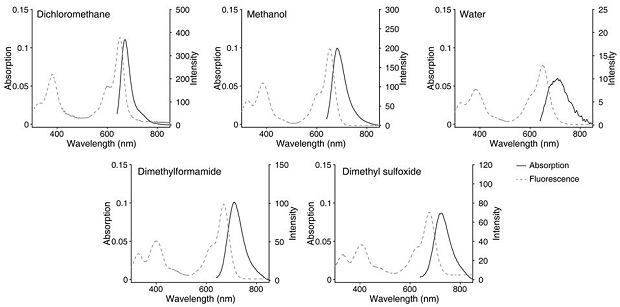
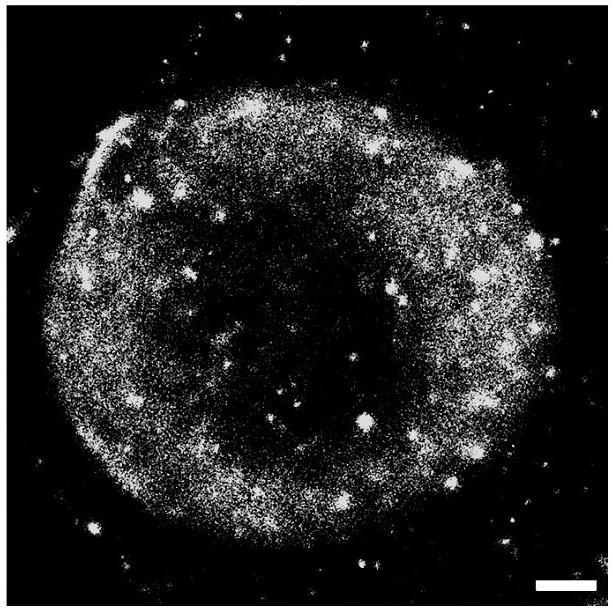
A class of fluborazole derivatives with cell membrane labeling function for single-molecule positioning super-resolution imaging and single-molecule tracking and applications
A technology with super-resolution imaging and labeling functions, applied in the field of fine chemical industry, can solve the problems of difficult to achieve high-density positioning super-resolution imaging, dyes cannot exhibit bright and dark state transition characteristics, etc., and is conducive to single-molecule tracking analysis, realization Long-range bright state, the effect of improving diagnostic accuracy and precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] Synthesis of 2,4,6,8-tetramethylfluoroboron pyrrole: synthesized according to the method in the literature, dissolving pyrrole in dry dichloromethane, adding benzoyl chloride dropwise in an inert gas atmosphere, and reacting in the dark 2-3h, after the reaction is complete, add triethylamine to adjust the pH to neutral, and then add boron trifluoride ether for coordination to obtain boron pyrrole. After the product is washed with water to remove boron trifluoride ether, pass column chromatography Isolation and Purification.
Embodiment 2
[0028]
[0029] Synthetic method: Dissolve the mixture of fluborazole (500mg, 1.54mmol) and p-methylpiperazine benzaldehyde (788mg, 3.88mmol) in toluene (50mL), add piperidine (3mL) in an inert gas atmosphere, and stir for 30min Afterwards, additional glacial acetic acid (3 mL) was added. Heating to reflux, using the water separator to separate the water produced in the reaction process, so that the reaction proceeds gradually to the right. Use TLC to detect the degree of completion of the reaction. After the conversion is complete (about 50-60 h), the reaction can be stopped and purified by column chromatography (dichloromethane:methanol=15:1) to obtain 706 mg of the product with a yield of 66.1%. The product structure was identified by HRMS, m / z=349.2036(z=2).
Embodiment 3
[0031]
[0032] Synthetic method: Dissolve bis(p-piperazinylphenyl)vinylfluoroborate (50 mg, 71.8 μmol) in 20 mL of N,N-dimethylformamide, add methyl iodide (447 μL, 7.18 mmol) dropwise, and stir at room temperature Reaction 48h. After completion of the reaction, wash with dichloromethane or n-hexane three times to obtain 52 mg of BDP1 (yield 99.7%). The product structure was identified by HRMS, m / z=363.2187 (z=2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com