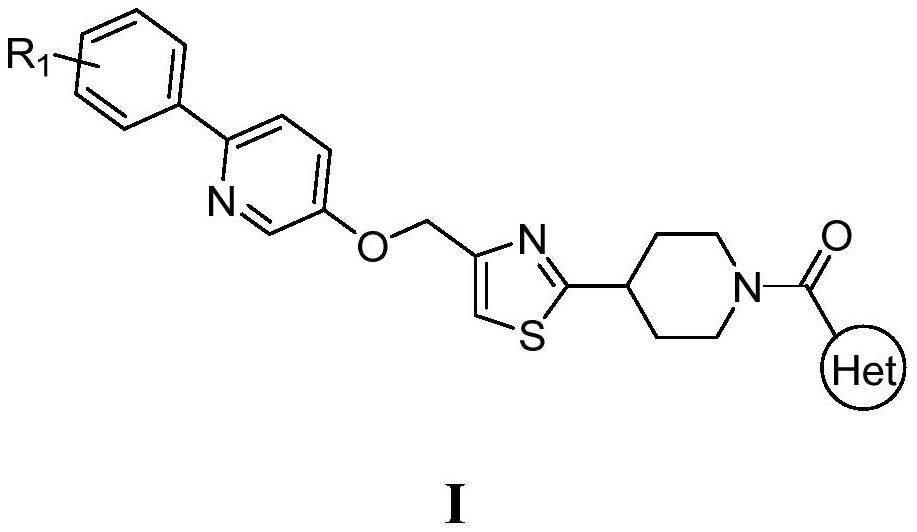
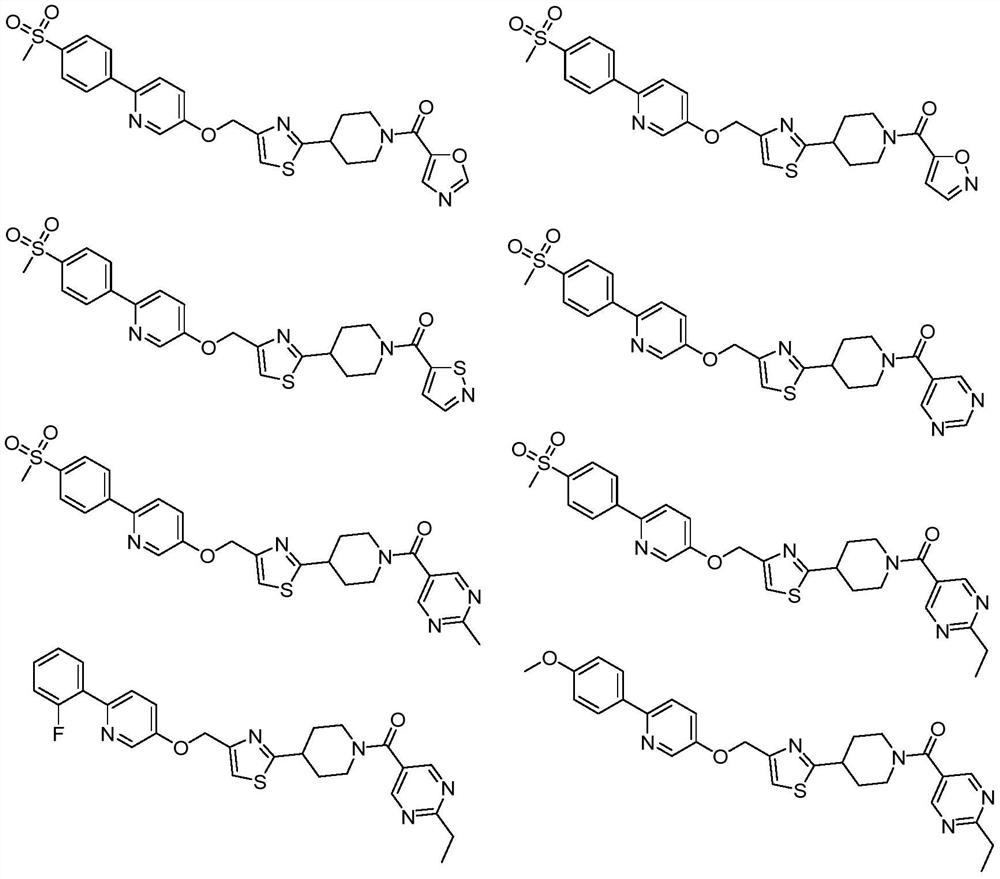
Novel heterocyclic amide piperidine derivative as well as preparation method and application thereof in hypoglycemic drugs
A kind of technology of heterocyclic amide piperidine and derivatives, applied in the field of drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
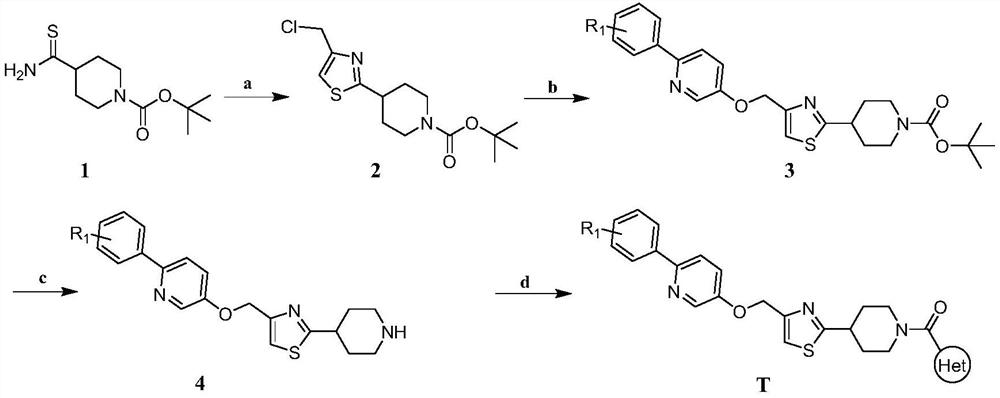
Embodiment 1
[0029] Synthesis of Example 1
[0030]
[0031] Synthesis of Intermediate 2:
[0032] Dissolve 4-aminothiocarbonyltetrahydropyridine-1(2H)-carboxylic acid tert-butyl ester (3.00g, 12.28mmol) and 1,3-dichloroacetone (1.71g, 13.51mmol) in toluene, heat up to Reflux reaction, after 10h TLC detects that the reaction is complete, concentrate under reduced pressure to remove toluene, then add 100mL ethyl acetate for extraction, wash the organic layer with water and saturated brine respectively, anhydrous Na 2 SO 4 Let dry overnight. The desiccant was filtered off, the solvent was evaporated by concentration under reduced pressure, and the residue was purified by silica gel column chromatography to obtain 3.01 g of the final product with a yield of 77.38%. 1 H NMR (400MHz, DMSO-d 6 )δ: 7.64(s, 1H), 4.78(s, 2H), 4.22(t, J=6.5Hz, 2H), 3.22-3.19(m, 1H), 2.91(t, J=12.0Hz, 2H,) ,1.70(dt,J=12.0Hz,3.0Hz,2H),1.40(d,J=5.1Hz,9H).
[0033] Synthesis of Intermediate 3
[0034] Intermed...
Embodiment 2
[0041]
[0042] 1 H NMR (400MHz, DMSO-d 6 )δ: 8.54(d, J=8.4Hz, 2H), 8.30(d, J=8.0Hz, 1H), 8.15(d, J=1.4Hz, 1H), 7.99-7.95(m, 3H), 7.64( s,1H),7.01(d,J=7.8Hz,1H),6.24(d,J=8.1Hz,1H),5.21(s,2H),4.23(d,J=12.1Hz,2H),3.40( s,3H),3.21-3.18(m,1H),2.90(t,J=12.0Hz,2H),1.76-1.71(m,2H).ESI-MS m / z:525.1[M+H] + .
Embodiment 3
[0044]
[0045] 1 H NMR (400MHz, DMSO-d 6 )δ:8.57-8.54(m,3H),8.14(d,J=2.4Hz,1H),7.98-7.94(m,3H),7.64(s,1H),7.30(d,J=8.0Hz,1H ),7.02(d,J=7.9Hz,1H),5.20(s,2H),4.24(d,J=12.1Hz,2H),3.38(s,3H),3.20-3.17(m,1H),2.91 (t,J=12.4Hz,2H),1.76-1.72(m,2H).ESI-MS m / z:541.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com