A kind of pyrrole alkaloid compound in purslane and its extraction and separation method
A separation method, the technology of purslane, applied in the direction of drug combination, organic chemistry, non-central analgesics, etc., can solve the problem of low structural novelty, and achieve the effect of environmentally friendly process method, simple and fast operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
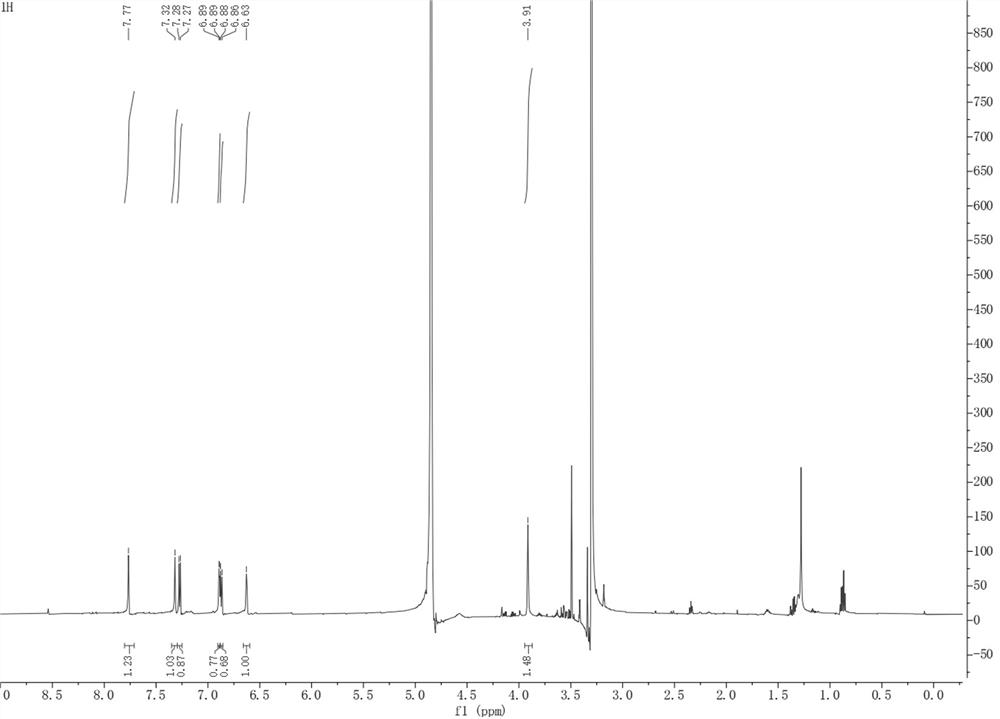
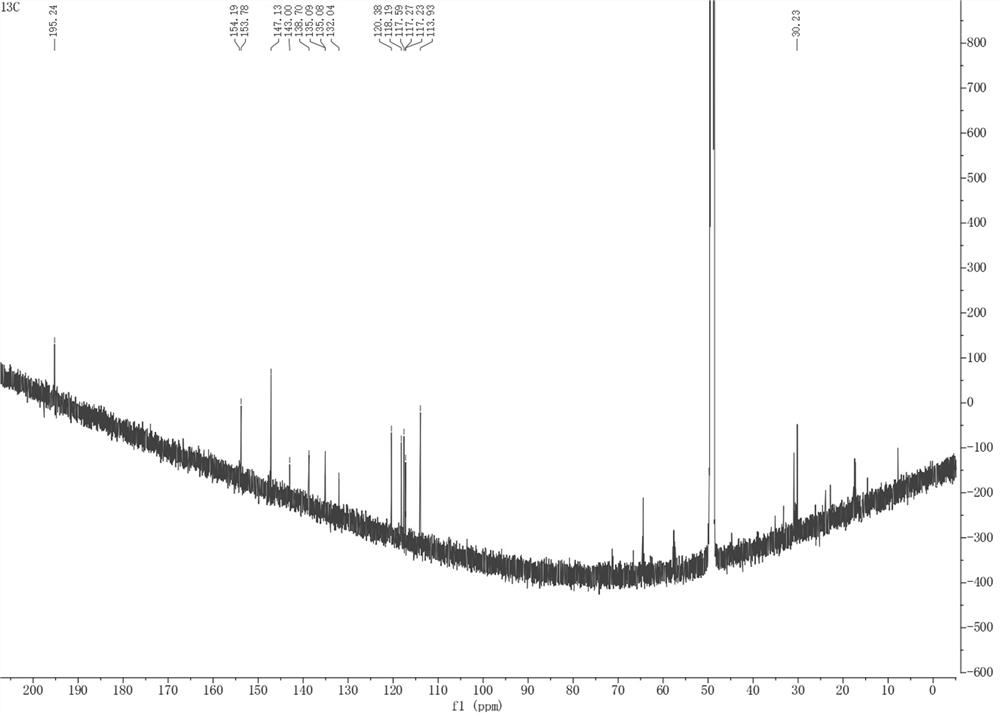
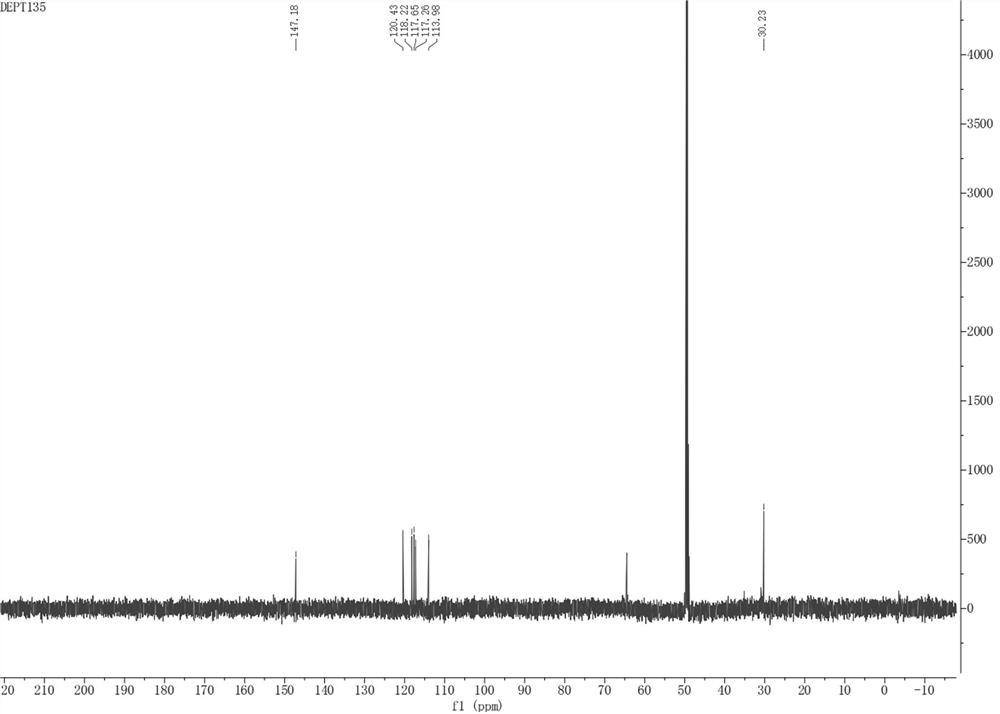
[0036] The present invention provides pyrrole alkaloid compound, molecular formula is C 16 h 9 NO 4 Named 10,11-dihydroxybenzo[5',6']pentaleno[1',2':3,4]pyrrolo[2,1-b]oxazol-7(11bH)-one, the chemical structure is:
[0037] .
[0038] The pyrrole alkaloid compound is named 10,11-dihydroxybenzo[5',6']pentaleno[1',2':3,4]pyrrolo[2,1-b]oxazol-7(11bH)- One, Table 1 is the NMR data of the pyrrole alkaloid compound: 1H-NMR with 13 C-NMR in MeOD- d 4 middle.
[0039] Table 1: NMR data of the compound 10,11-dihydroxybenzo[5',6']pentaleno[1',2':3,4]pyrrolo[2,1-b]oxazol-7(11bH)-one of the present invention
[0040] .
[0041] The structure of the present invention pyrrole alkaloid compound 10,11-dihydroxybenzo[5',6']pentaleno[1',2':3,4]pyrrolo[2,1-b]oxazol-7(11bH)-one identification and derivation.
[0042] 10,11-dihydroxybenzo[5',6']pentaleno[1',2':3,4]pyrrolo[2,1-b]oxazol-7(11bH)-one: The compound is yellowish brown powder, easily soluble in Methanol. UHPLC-ESI-Q-TOF-M...
Embodiment 2
[0051] Example 2 Anti-inflammatory effect of pyrrole alkaloid compounds of the present invention.
[0052] 1 main material.
[0053] 1.1. Drugs and reagents: The compounds used in the experiment were prepared by the above method with a purity of ≥98%, weighed accurately, and diluted with DMSO to the required solutions for the following dosage groups. Fetal bovine serum (U.S. Hyclone Company); penicillin, streptomycin (Hangzhou Sijiqing Company); DMSO (U.S. Sigma Company); cck-8 kit (Wuhan Boster Bioengineering Co., Ltd.); DMEM high glucose medium, LPS , ELISA kits for IL-1β, TNF-α (Soleibao Technology Co., Ltd.); cell lysate.
[0054] 1.2 Cell line: RAW264.7 macrophages (US ATCC cell bank).
[0055] 1.3 Grouping: control group, LPS group and experimental group, each group.
[0056] 2 Experimental methods.
[0057] 2.1 Cell culture, DMEM high-glucose medium, add 10% fetal bovine serum, 1% antibiotics (100U / mL penicillin and 100μg / mL streptomycin), place at 37°C, 5% CO 2 cu...
Embodiment 3
[0070] Example 3 Anticholinesterase effect of compounds of the present invention.
[0071] 1. Main materials.
[0072] 1.1. Drugs and reagents: The pyrrole alkaloid compounds used in the experiment were prepared by the above method, with a purity of 90-99%. Technology), phosphorus 5,5'-dithiobis (2-nitrobenzoic acid) (Dithiobisnitrobenzoic acid, DTNB, Shanghai Jinsui Biotechnology Co., Ltd.), acetylcholinesterase (AChE) and iodide thioacetylcholine (Acetylthiocholine iodide, ATCI, Dalian Meilun Biotechnology Co., Ltd).
[0073] 1.2. Grouping: divided into blank group, control group and sample group.
[0074] 2 Experimental methods.
[0075] 2.1 Sample preparation.
[0076] Precisely weigh the sample and 0.11 mg of physostigmine, respectively, and use methanol as a solvent to prepare five gradient concentrations of 2.5 μM, 5.0 μM, 10.0 μM, 20.0 μM and 40.0 μM. Accurately weigh 7.8005g of sodium dihydrogen phosphate and 17.9070g of disodium hydrogen phosphate, and adjust th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com