A kind of bacterium and mof base carrier hybrid material and its preparation method and application
A hybrid material and MOF technology, which is applied in the field of hybrid materials of bacteria and MOF-based carriers and their preparation, can solve the problems of single treatment mode and poor accuracy of traditional nano-therapeutics, so as to enhance the curative effect of tumor treatment and improve the efficiency of tumor inhibition. , the effect of reducing off-target side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
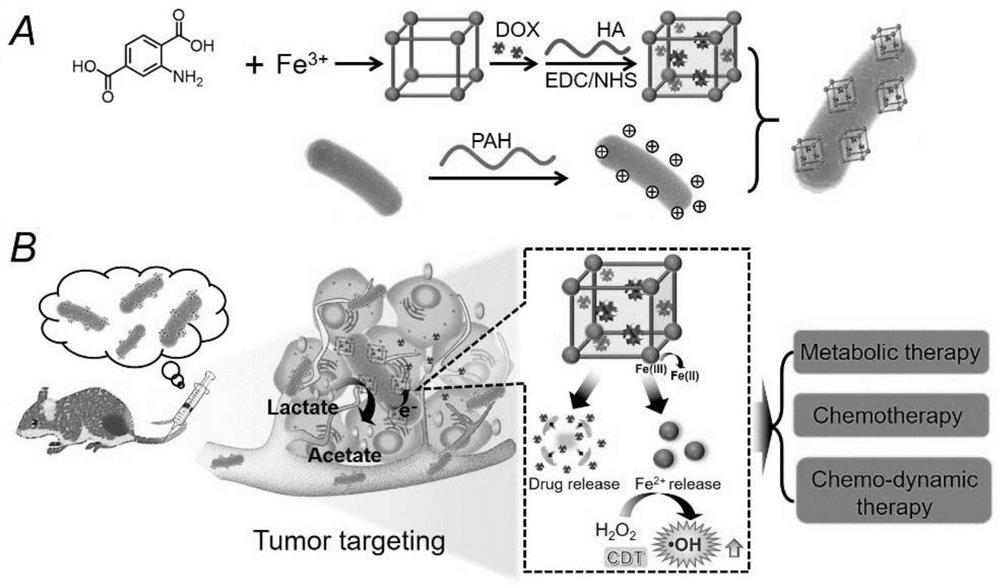
[0045] According to another exemplary embodiment of the present invention, there is provided a method for preparing a hybrid material of a bacterium with a MOF-based carrier, such as Figure 6 As shown, the method comprises:
[0046] S1, moF nanoparticles loaded with therapeutic agents, to obtain a load of chemotherapy drugs MOF carrier;
[0047] As an alternative embodiment, the MOF nanoparticles are synthesized using controlled titration, comprising:
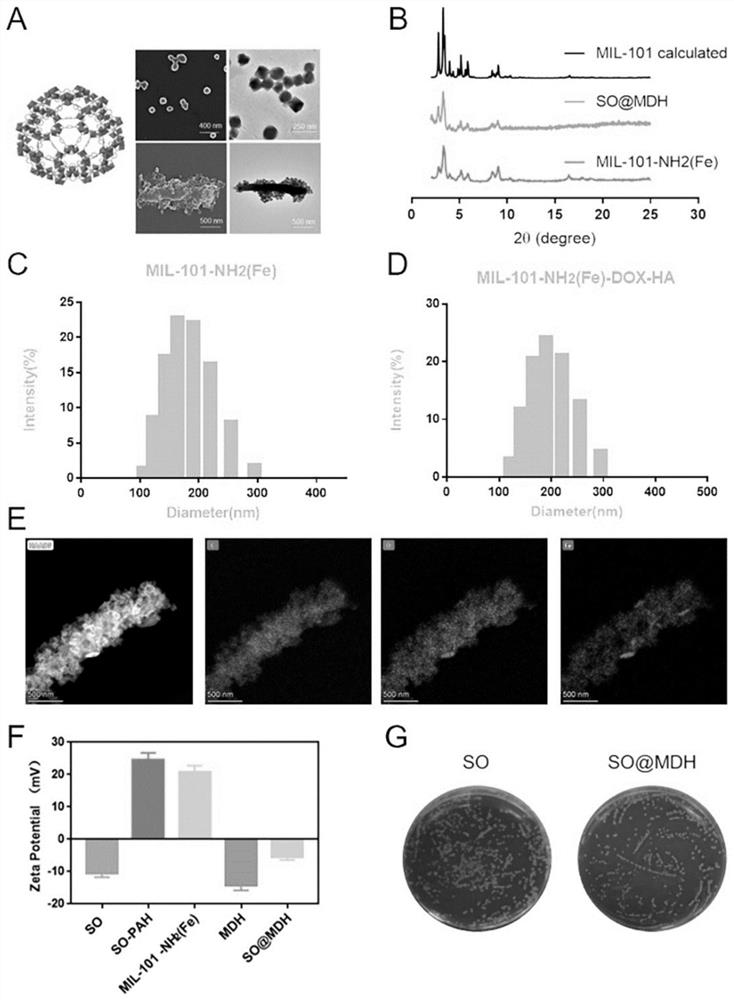
[0048] MIL-101-NH was synthesized by controlled titration 2 (Fe) MOF, as follows: the 10~ 50mM metal precursor solution and 10~50mM ligand precursor solution were added to the reactor containing 8~16mL DMF / water mixed solvent by the syringe pump at 10~30mL / h through a separate pipeline, and the reaction was carried out under stirring at 50 °C. After the reaction is completed, the reaction solution is removed, centrifuged (10000~12000rpm, 10~20min), washed to obtain MIL-101-NH with morphology and regular size 2 (Fe) MOF, i.e. the MO...
Embodiment 1
[0089] Example 1 Khiva bacteria bind iron-based MOF hybrid active material and preparation method thereof
[0090] (1) The metal precursor solution (40mM of aqueous iron chloride solution) and the ligand precursor solution (40mM of 2-amino-terephthalic acid DMF / water (V / V = 1 / 1) solution) are configured, and then the above-described precursor solution is added by the syringe pump through a separate pipeline at 20mL / h to the reactor containing 10mL DMF / water (1 / 3) mixed solvent, and the reaction is 24h under stirring at 50 ° C. Subsequently, centrifugation (11,000 rpm, 15min) and washing yielded MIL-101-NH with morphology and dimensions 2 (Fe) MOF。
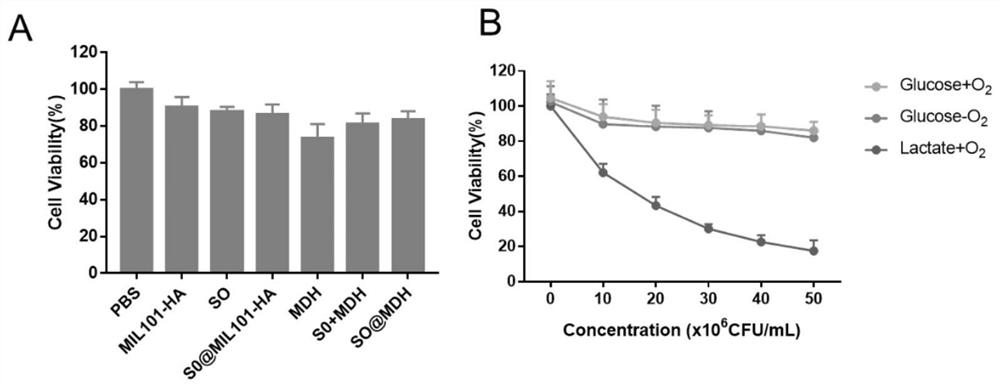
[0091] (2) The above (1) yielded 10mg MIL-101-NH 2 (Fe) MOF is evenly dispersed in 10 mL aqueous solution and sonicated for 10 min to make it fully dispersed. Subsequently, an aqueous solution of 1 mL DOX (0.5 mg / mL) was added to the above-mentioned nano MOF dispersion, stirred at room temperature to avoid light for 24 h, an...
Embodiment 2
[0096] Example 2 Khiva bacteria bind iron-based MOF hybrid active material and preparation method thereof
[0097] (1) The metal precursor solution (10mM aqueous iron chloride solution) and the ligand precursor solution (10mM of 2-amino- terephthalic acid DMF / water (V / V = 1 / 1) solution) are configured), and then the above-described precursor solution is added by the syringe pump through a separate pipeline at 10mL / h to a reactor containing 8mLDMF / water (1 / 3) mixed solvent, and the reaction is 24h at 50 °C stirring. Subsequently, centrifugation (11,000 rpm, 15min) and washing yielded MIL-101-NH with morphology and dimensions 2(Fe) MOF。
[0098] (2) The above (1) resulted in 5 mg MIL-101-NH 2 (Fe) MOF is evenly dispersed in 10 mL aqueous solution and sonicated for 10 min to make it fully dispersed. Subsequently, an aqueous solution of 1 mL DOX (0.1 mg / mL) was added to the above-mentioned nano MOF dispersion, stirred at room temperature to avoid light for 24 h to obtain MIL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com