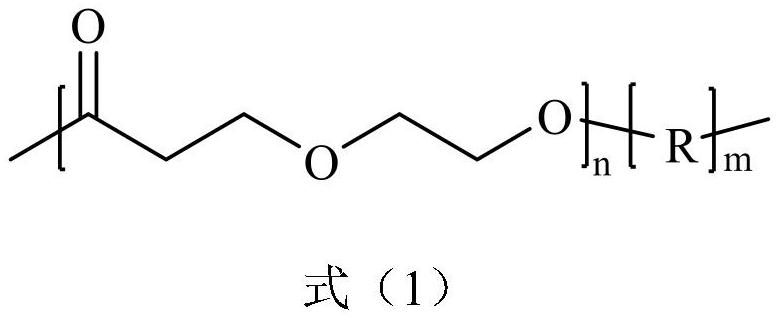
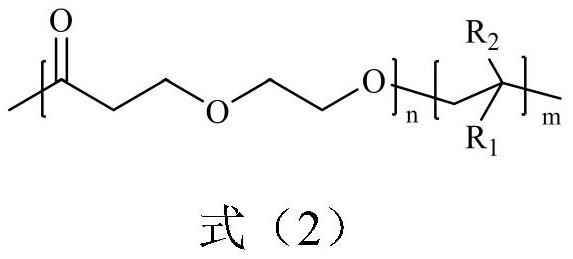
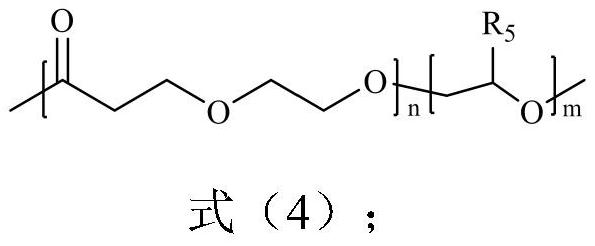
A poly(1,5-dioxepan-2-one)-based block polymer, its preparation method and application
A technology of dioxetane and block polymer is applied in the field of poly-based block polymer and its preparation, which can solve the problems of inability to meet the ionic conductivity of electrolytes, low ionic conductivity and the like, and improve mechanical properties. and thermal stability, simple preparation process, quantifiable production effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 1. First, heat and vacuum the Shrank bottle with magnetron, replace nitrogen three times back and forth, and remove water and air. Quickly add 1,5-dioxepan-2-one (DXO) (3.48 g, 30 mmol), benzyl alcohol (BnOH) (108 mg, 1 mmol), 5 mL THF under nitrogen, "freeze vacuum-thaw" Three times, 1,8-diazabicycloundec-7-ene (DBU) (152 mg, 1 mmol) was then added under nitrogen. The reaction was carried out at 25 °C for 6 h, and a small amount of hydrochloric acid / methanol mixed solvent was added to terminate the reaction. Excess diethyl ether was used as a precipitant and dried at room temperature to obtain poly(1,5-dioxepane-2-one homopolymer) ( PDXO 30 ).
[0058] 2. Insert the PDXO 30 (3g, 1mmol), 10mL CH 2 Cl 2 A three-necked flask placed in a -5°C low temperature reactor was added, and 2-bromoisobutyryl bromide (345 mg, 1.5 mmol) was dissolved in 5 mL of CH 2 Cl 2 and added dropwise to the above three-necked flask, after the dropwise addition, the reaction was carried ou...
Embodiment 2
[0066] The difference from Example 1 is: the amount of PS described in step 3 is (5.20 g, 50 mmol), and other implementation conditions are the same as those in Example 1 to synthesize PDXO 30 -b-PS 30 Block polymer electrolytes are the same to make PDXO 30 -b-PS 50 Block polymer electrolytes. By analyzing the polymer electrolyte, we get M n =8000g / mol of PDXO 30 -b-PS 50 A block polymer electrolyte with a block PDXO:PS molar ratio of 3:5.
Embodiment 3
[0068] The difference from Example 1 is: the amount of PS described in step 3 is (1.04 g, 10 mmol), and other implementation conditions are the same as those in Example 1 to synthesize PDXO 30 -b-PS 30 Block polymer electrolytes are the same to make PDXO 30 -b-PS 10 Block polymer electrolytes. By analyzing the polymer electrolyte, we get M n =4000g / mol of PDXO 30 -b-PS 10 A block polymer electrolyte with a block PDXO:PS molar ratio of 3:1.
[0069] The performance of the block polymer electrolyte obtained in Examples 1-3 is characterized, and the test results are shown in Table 1. It can be seen from Table 1 that in the PDXO-b-PS block polymer electrolyte, with the increase of the PDXO block As the content increases, the room temperature ionic conductivity of the electrolyte also increases, indicating that the PDXO block in the PDXO-b-PS block polymer electrolyte is beneficial to Li + Transmission.
[0070] Table 1 Properties of the block polymer electrolytes obtained ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com