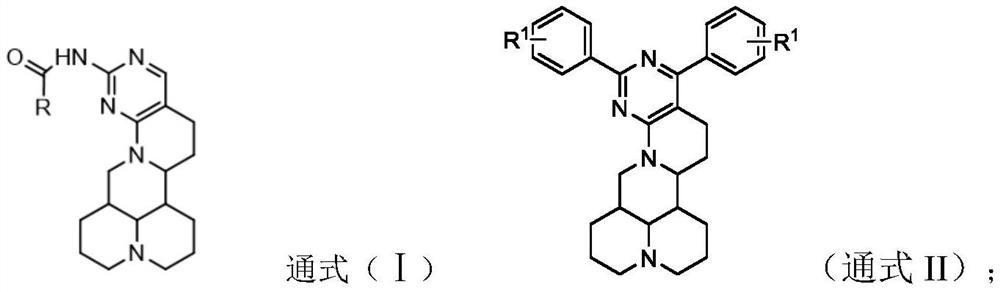
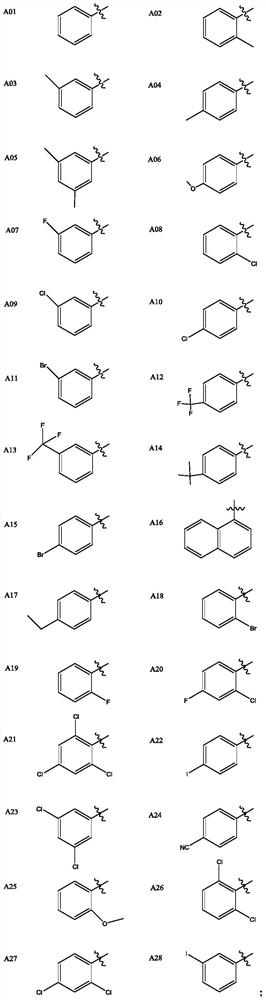
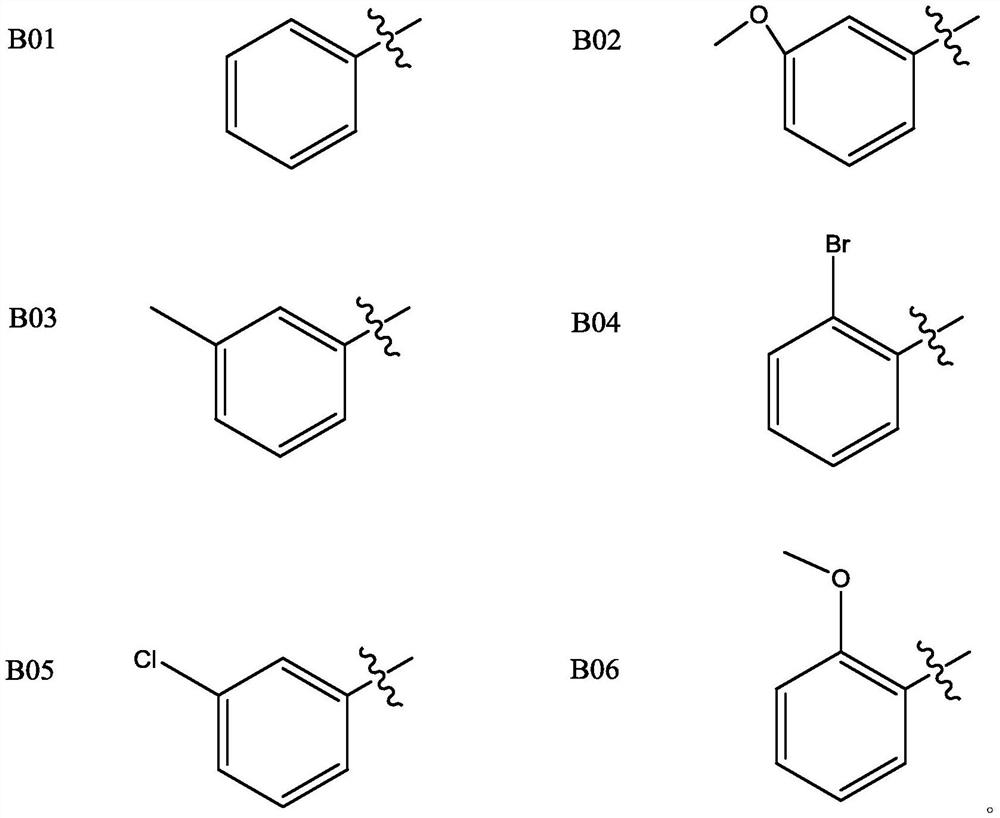
Matrine pyrimidine derivative, and preparation method and application thereof
A technology for matrine and pyrimidine, which is applied in the field of matrine and pyrimidine derivatives and the preparation thereof, can solve the problems of lack of matrine and pyrimidine compounds, and achieves the effects of inhibiting proliferation and differentiation and having a simple and convenient preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Synthesis of intermediate 14-formyl-15-chloromatrine
[0032] Weigh 20g (80mml, 1eq) of matrine and place it in a 150ml dry beaker, add 120ml of dichloromethane and stir to fully dissolve the matrine, and place it in a dry environment for later use after dissolving. Prepare a 500ml dry three-necked flask, seal it, evacuate it and protect it with nitrogen, and pre-cool the three-necked flask under an ice bath. Weigh 17.52g of DMF, and inject DMF into a three-neck flask. Then weigh 36.8g of phosphorus oxychloride and slowly add it dropwise to DMF. After the dropwise addition, react at 0°C for 1h, and finally add the dissolved matrine to the reaction solution. At this time, the reaction solution changes from a colorless liquid Turned into reddish brown, remove the ice bath, let the reaction solution slowly rise to room temperature and react for 24h to terminate the reaction. After the reaction, first remove DCM and part of phosphorus oxychloride by rotary evaporator,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com