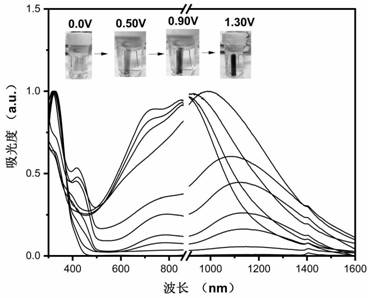
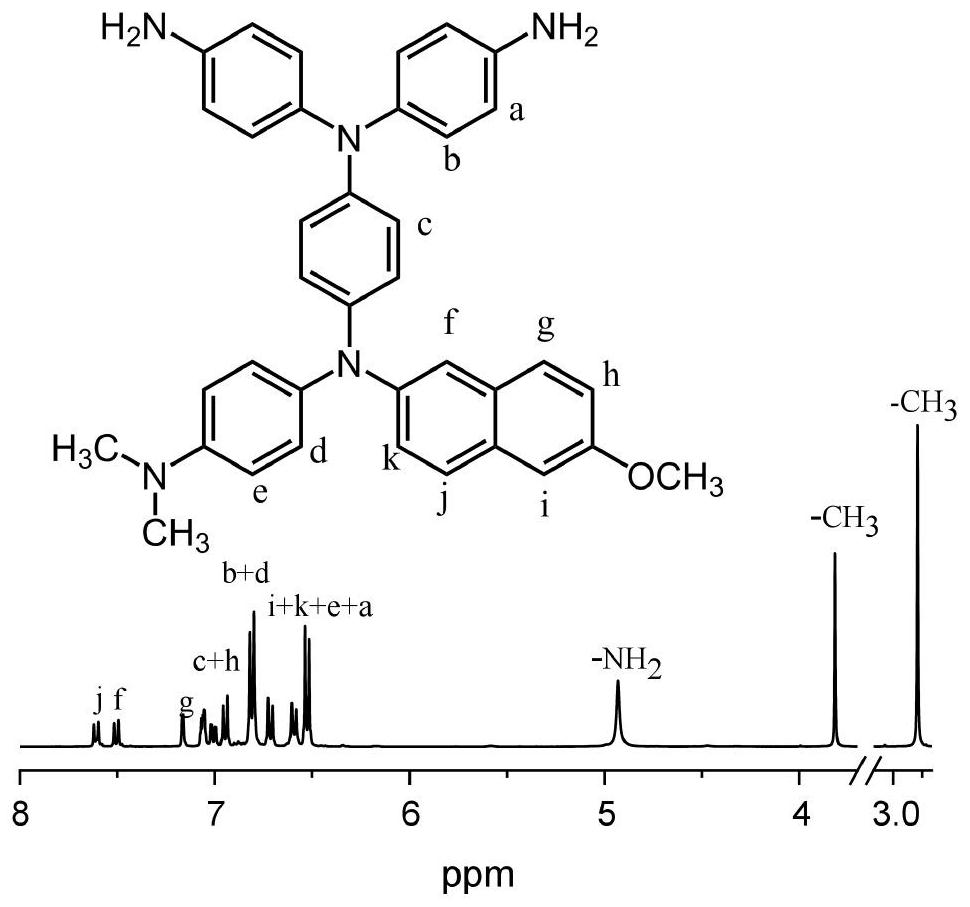
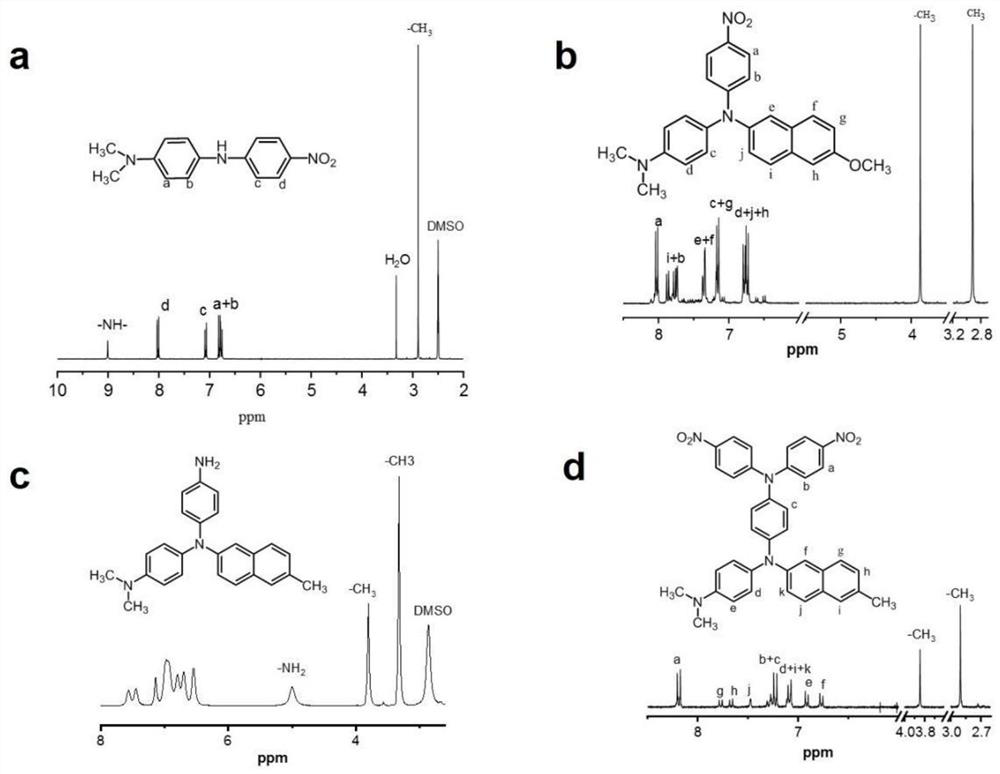
A kind of diamine compound containing three n centers, preparation method and application thereof, polyamide and preparation and application thereof
A technology of amine compounds and polyamides, applied in the field of polyamides and its preparation and application, can solve the problems of unfavorable electrochemical stability, poor electrical activity, high oxidation potential of single cation radicals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The present invention provides the preparation method of the diamine compound containing three N centers described in the above technical scheme, comprising the following steps:
[0024] Mixing p-halogen nitrobenzene, N,N-dimethyl-p-phenylenediamine, a basic catalyst and a first polar solvent, and carrying out a first substitution reaction to obtain the diphenylamine derivative of the structure shown in formula II;
[0025] Mixing the diphenylamine derivative, the brominated fluorophore compound, the copper catalyst, the co-catalyst, the phase transfer catalyst and the second polar solvent to carry out a coupling reaction to obtain the mononitro compound of the structure shown in formula III;
[0026] Mixing the mononitro compound, the palladium carbon catalyst, the hydrazine hydrate and the first mixed solvent to carry out the first reduction reaction to obtain the monoamino compound of the structure shown in formula IV;
[0027] Mixing the monoamino compound, p-haloge...
Embodiment 1
[0082] Preparation of 4-N,N-dimethyl-4'nitrodiphenylamine:
[0083]Under nitrogen protection, add 27.2g (200mmol) of N,N-dimethyl-p-phenylenediamine, 25.4g (180mmol) of p-fluoronitrobenzene and 18.2g (180mmol) of triethylamine into a 500mL there-necked flask, add 200mL DMSO, the solid content of the mixed system was 24.3wt%, reacted at 85°C for 20 hours, discharged into ice water, dried to obtain dark red solid crude material, and the crude material was mixed with ethanol and DMF with a volume ratio of 4:1 The liquid was recrystallized to obtain 42 g of purple crystals with a yield of 91%. The structural formula is:
[0084]
[0085] Under nitrogen protection, 14.9 g (58.0 mmol) of the 4-N,N-dimethyl-4'nitrodiphenylamine, 10.0 g (48.3 mmol) 2-bromonaphthalene, 46.0 g (241.5 mmol) of the iodinated Cuprous, 69.2g (193.2mmol) of cesium carbonate and 12.2g (33.8mmol) of dibenzo-18-crown ether-6 were added in a 500mL there-necked flask, 227mL of dichlorotoluene was added, and t...
Embodiment 2
[0093] Under nitrogen protection, 10.4g (40.5mmol) of 4-N,N-dimethyl-4'nitrodiphenylamine prepared in Example 1, 8.0g (33.7mmol) of 2-bromo-6methoxynaphthalene, 10.7 g (168.5 mmol) of copper powder, 18.6 g (134.8 mmol) of potassium carbonate and 5.3 g (20.2 mmol) of 18-crown-6 were added to a 250 mL three-necked flask, 75.0 mL of dichlorotoluene was added, and the solid content of the mixed system was 36wt%, react at 175°C for 20h, filter while hot to obtain a filtrate, remove the dichlorotoluene solution by distillation under reduced pressure, and use a dichloromethane / petroleum ether developing agent with a volume ratio of 2.5:1 to carry out the column layer of the solid crude material. analytical and purification to obtain 9.3 g of a red mononitro compound 4-nitrophenyl-4-N,N dimethylphenyl-2-amino-2-bromo-6-methoxynaphthalene with a yield of 56.0% , the structure is as follows:
[0094]
[0095] Under nitrogen protection, in a 250mL there-necked flask, 8.0g (19.3mmol) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com