2-(2-Hydroxyphenyl) benzothiazole derivatives and their preparation methods and their application in fluorescent anti-counterfeiting
A benzothiazole and hydroxyphenyl technology, which is applied in the field of fluorescent materials and anti-counterfeiting applications, can solve the problems of high fluidity of fluorescent ink, fast attenuation of fluorescent intensity, low viscosity of fluorescent ink, etc., and is convenient for large-scale application and promotion. Stable and fast response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
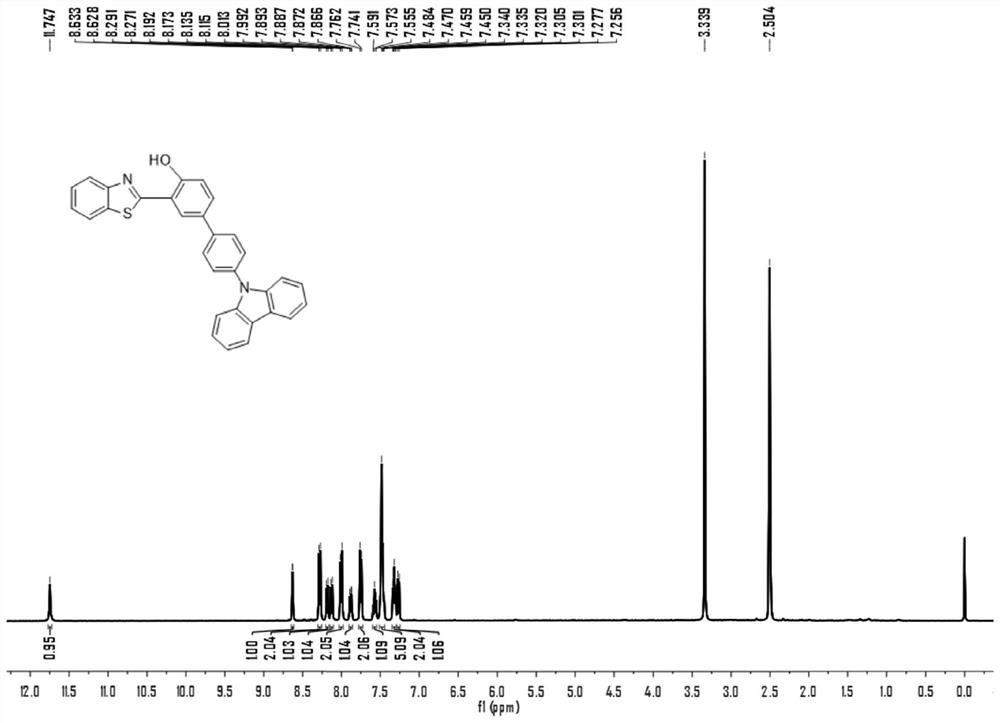
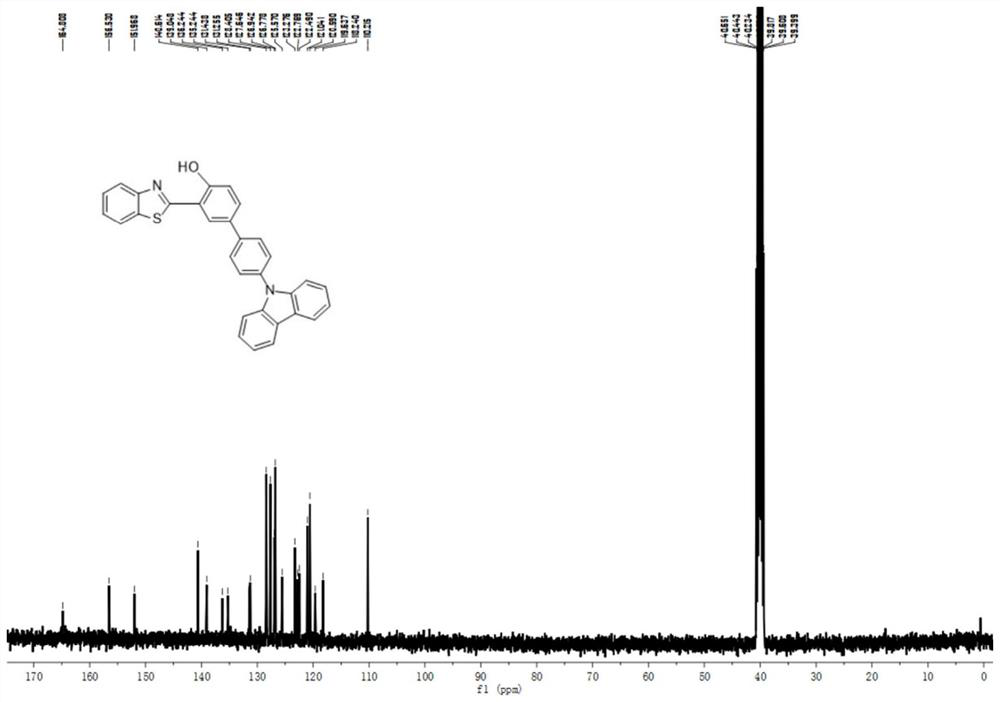
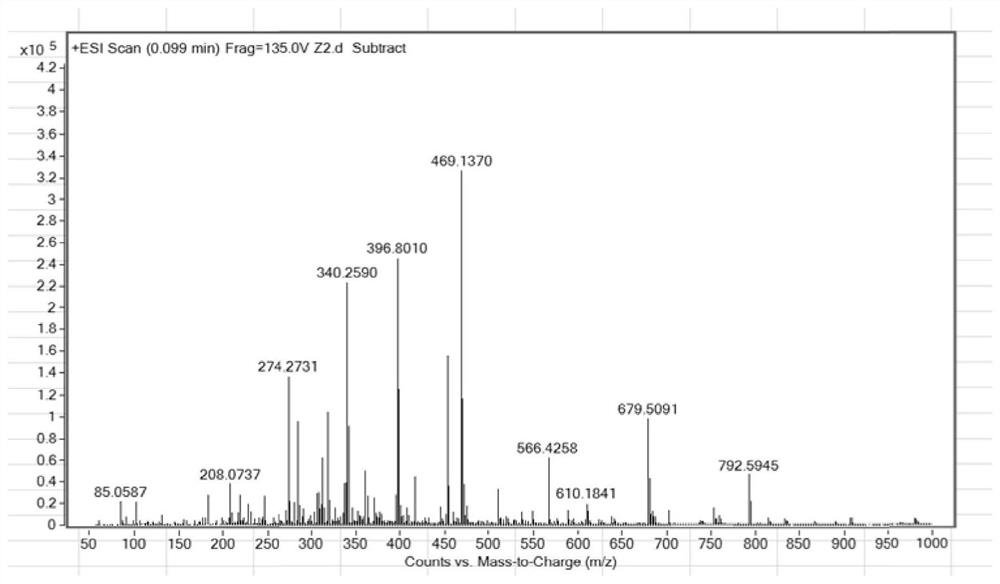
[0046] Example 1. Preparation of 2-(2-hydroxyphenyl) benzothiazole derivatives
[0047] In a 25 mL round bottom flask, 306 mg (1 mmol) of 2-benzothiazolyl-4-bromophenol was dissolved in 10 mL of tetrahydrofuran, and then 2 mL of potassium carbonate aqueous solution (concentration 2 mM) was added under nitrogen atmosphere. Next, 288 mg (1 mmol) of 4-(9-carbazolyl) benzeneboronic acid was added to the reaction mixture, and after stirring at room temperature for 10 minutes, Pd(PPh 3 ) 4 (58 mg, 0.05 mmol) was added to the reaction solution. After the mixture was stirred at 80°C for 8 hours, 5 mL of purified water was added to quench the reaction. Extracted with dichloromethane three times, the combined organic layers were washed with water and dried over sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and left to crystallize to obtain 270 mg of orange-yellow 2-(2-hydroxyphenyl)benzothiazole derivative solids in a yield of 270 mg. was 57.6%.
[...
Embodiment 2
[0056] Example 2. Application of 2-(2-hydroxyphenyl) benzothiazole derivatives in anti-counterfeiting
[0057] (1) preparing a tetrahydrofuran solution of 2-(2-hydroxyphenyl)benzothiazole derivative with a concentration of 50 μM to obtain an anti-counterfeiting solution;
[0058] (2) Arrange the anti-counterfeiting liquid on the anti-counterfeiting commodity, and after drying, irradiate with 365nm ultraviolet light, and the part where the anti-counterfeiting substance is arranged emits yellow fluorescence;
[0059] (3) Place the anti-counterfeiting product in an amine vapor atmosphere for 30 seconds, then irradiate it with 365nm ultraviolet light, and the part where the anti-counterfeiting substance is set emits blue-green fluorescence;
[0060] (4) The anti-counterfeiting goods are smoked in an acetic acid vapor atmosphere for 30 seconds to restore the yellow fluorescence and realize the reversible response of solid-state fluorescence.
experiment example 1
[0061] Experimental Example 1: Testing of Sensing Properties of 2-(2-Hydroxyphenyl)benzothiazole Derivatives
[0062] Test method: Add 10 μL of 50 μM tetrahydrofuran solution of 2-(2-hydroxyphenyl)benzothiazole derivatives to a 0.8cm×1.5cm filter paper strip, and then dry it in the air for 24 hours to prepare Filter paper strips loaded with fluorescent dyes.
[0063] Amine vapor was prepared by placing isopropylamine (15 μL) in a 1 cm×1 cm×4 cm quartz cuvette, sealed, and left at room temperature for 30 minutes to saturate.
[0064] The dye-loaded filter paper strips were mounted on a glass plate, which was placed in a quartz cuvette. Place the cuvette with the glass plate mounted on the solid sample holder of the fluorescence spectrometer and test its fluorescence spectrum. The filter paper strip was placed in isopropylamine vapor for 30 seconds and its fluorescence spectrum was tested. The filter paper strip was taken out, dried with a hair dryer for 1 minute, then placed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com