A kind of diamine compound containing symmetrical double fluorophore structure and its preparation and application, polyamide and polyimide and its preparation and application
A technology of dual fluorophores and amine compounds, which is applied in the field of electronically controlled fluorescence, can solve the problems of long transition time, high oxidation potential, and low fluorescence contrast, and achieve the effects of enhanced dissolution and film-forming ability, enhanced fluorescence intensity, and high fluorescence contrast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The present invention provides a method for preparing a diamine compound containing a symmetrical double fluorophore structure according to the above technical solution, comprising the following steps:
[0030] Mixing p-phenylenediamine, p-fluoronitrobenzene, a first catalyst and an organic solvent to carry out a substitution reaction to obtain a dinitro monomer;
[0031] Mixing the dinitro monomer, the brominated fluorescent compound, the second catalyst and the polar solvent, and carrying out a coupling reaction to obtain a dinitro compound;
[0032] The dinitro compound, the third catalyst, the reducing agent and the mixed solvent are mixed, and a reduction reaction is carried out to obtain the diamine compound containing the symmetrical double fluorophore structure of the structure shown in formula I;
[0033] The dinitro monomer has the structure shown in formula II-1:
[0034]
[0035] The structural formula of the brominated fluorescent compound is Br-R;
[...
Embodiment 1
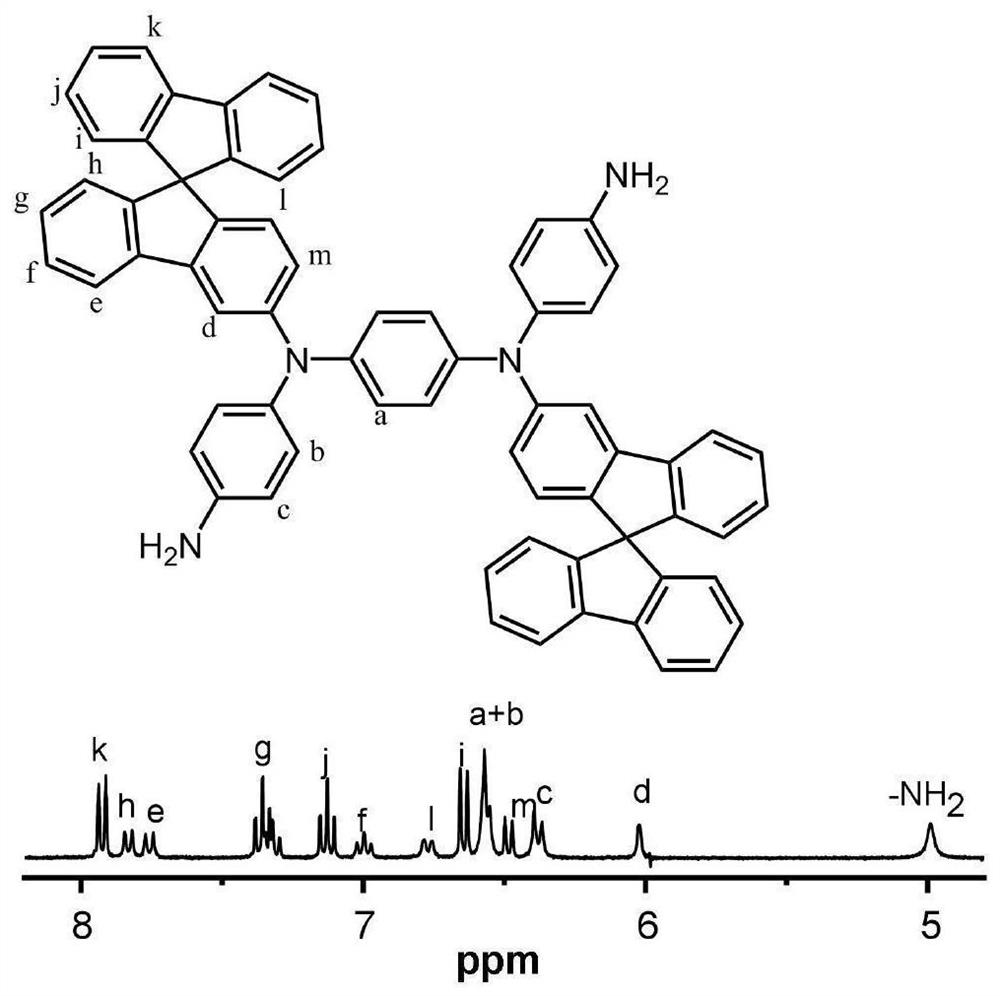
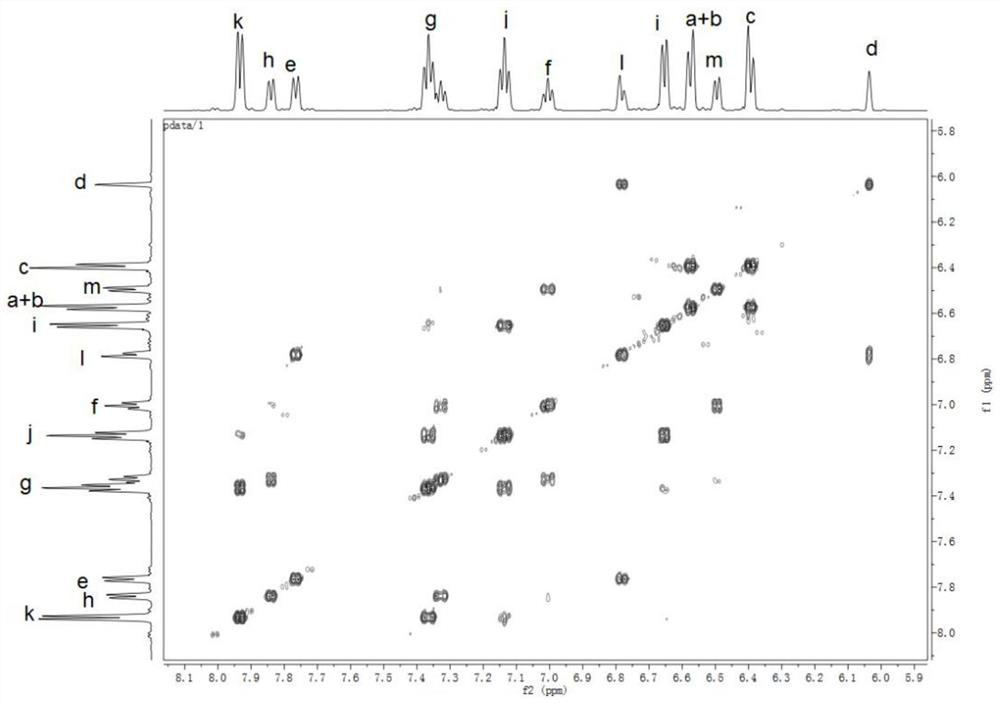
[0099] The preparation of monomer N,N'bis(4-nitrophenyl)-1,4-p-phenylenediamine, its structure is:
[0100]
[0101] Under nitrogen conditions, add 21.6g (200mmol) of p-phenylenediamine, 64.9g (460mmol) of p-fluoronitrobenzene, 101.2g (1000mmol) to the 1000mL there-necked flask equipped with a mechanical stirring device, a thermometer and a condenser tube. Triethylamine and 45.1 g (140 mmol) of tetrabutylammonium bromide, then 424 mL of sulfolane was added, the solid content of the obtained system was 30.3 wt%, and the reaction was carried out at 180 ° C under microwave conditions for 7 h, and the obtained product system was cooled to room temperature and then discharged. In 2000mL of ice water, suction filtration and drying to obtain a reddish-brown crude material, the obtained crude material is recrystallized three times with a DMSO / ethanol solution with a volume ratio of 1:3 to obtain 29.4g of purple-red N,N'-bis(4- Nitrophenyl)-1,4-p-phenylenediamine powder, 42% yield. ...
Embodiment 2
[0107] The preparation of diamine monomer N,N'-bis(4-aminophenyl)-N,N'-bis(9,9 spirobifluorenyl)-1,4-p-phenylenediamine, its structure is:
[0108]
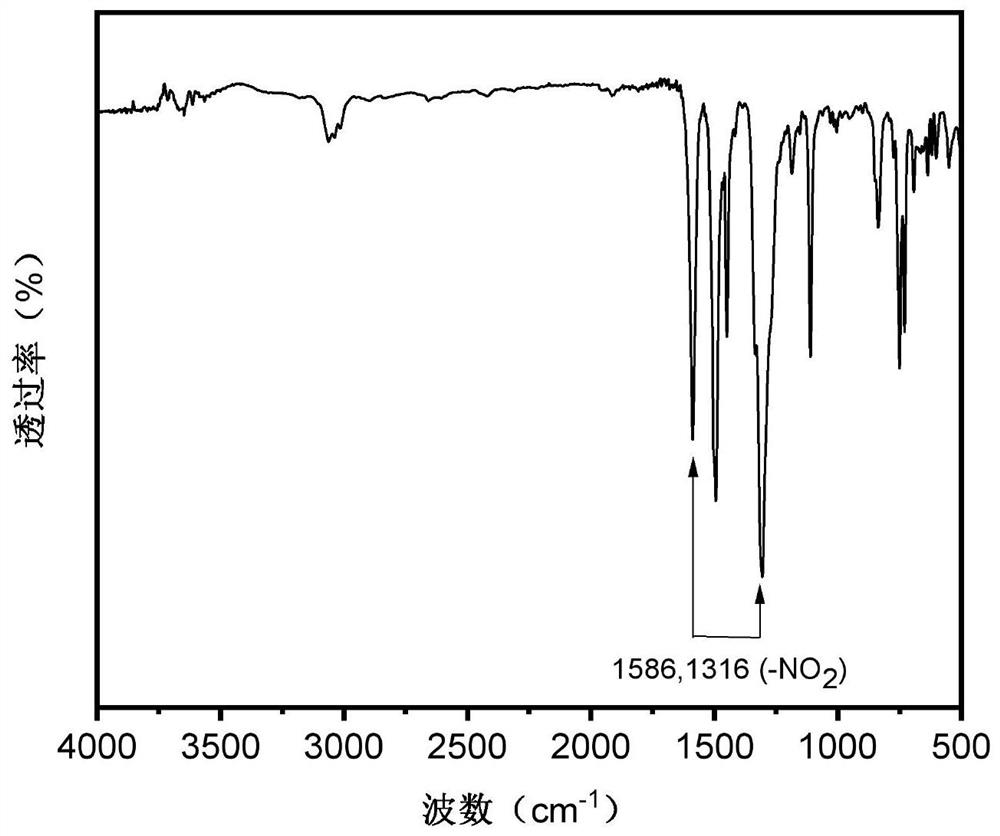
[0109] Under a nitrogen atmosphere, 4.2g (12mmol) N,N'-bis(4-nitrophenyl)-1,4 prepared in Example 1 was added to a 250mL three-necked flask with a mechanical stirring device, a thermometer and a condenser tube. - p-phenylenediamine, 9.8g (24.8mmol) 2-bromo-9,9'spirobis(9H-fluorene), 3.8g (60mmol) copper powder, 13.2g (96mmol) potassium carbonate and 1.9g (7.2mmol) 18 crown ether-6, then add 50mL o-dichlorobenzene, the solid content of the obtained system is 33.5wt%, react at 160 ° C for 24h, filter while hot to remove copper powder and salt, remove the filtrate by vacuum distillation, and use the volume ratio of 2:1 dichloromethane / petroleum ether was used as a developing solvent to carry out column chromatography to obtain 6.1 g of orange-yellow dinitro compound with a yield of 52.0%;
[0110] Under nitrogen protective atmos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com