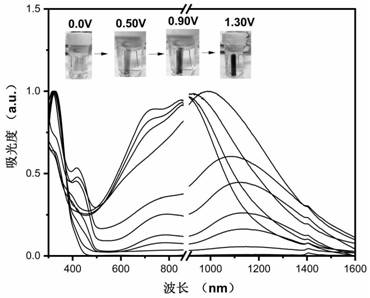
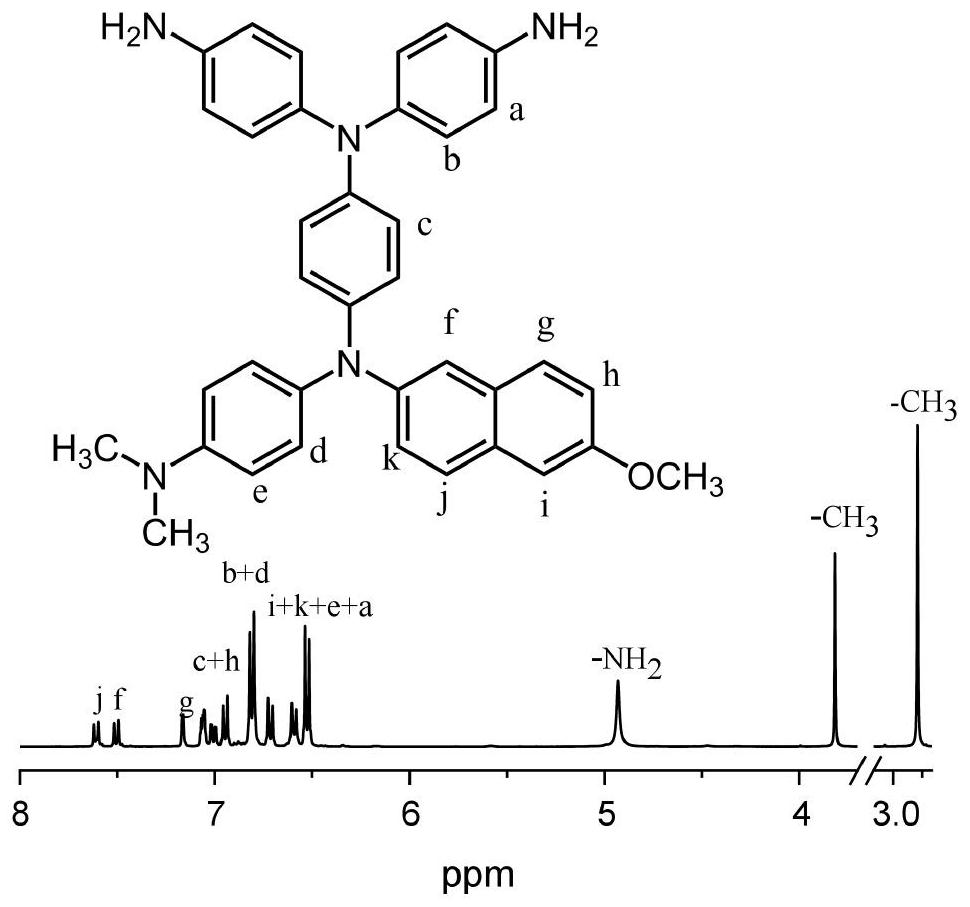
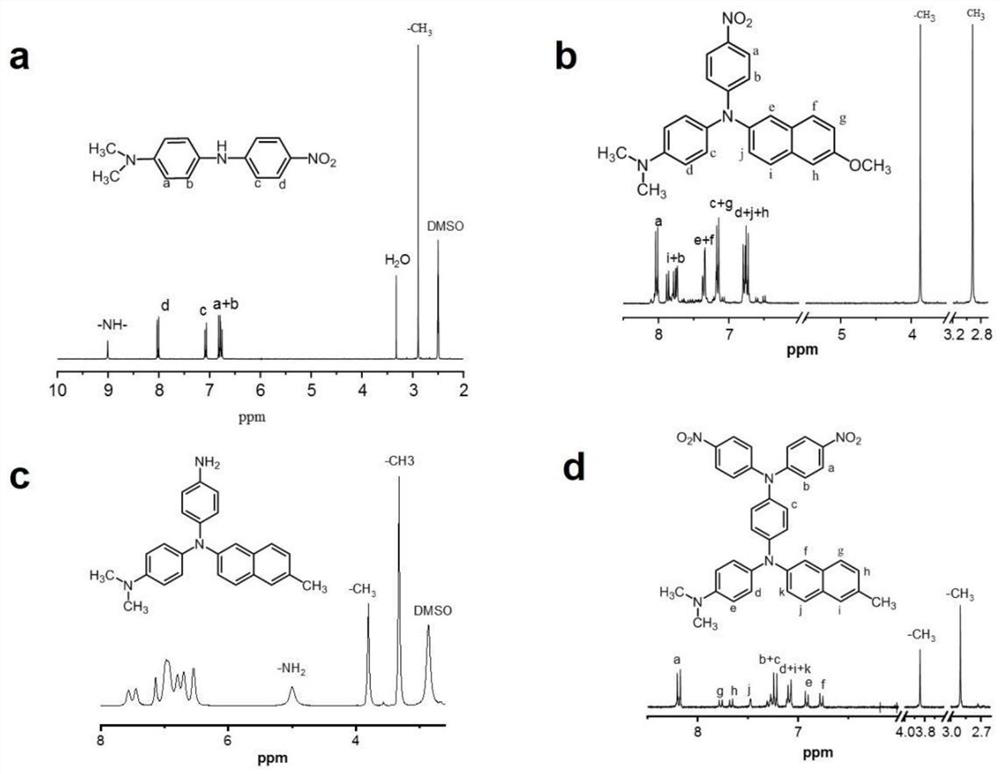
Diamine compound containing three N centers, preparation method and application of diamine compound, polyamide, and preparation and application thereof
A technology of amine compound and polyamide, which is applied in the field of polyamide and its preparation and application, can solve the problems of high oxidation potential of single cation free radicals, instability of dications, unfavorable development and utilization of multi-color photoelectric materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The present invention provides a preparation method of a diamine compound containing three N centers described in the above technical scheme, comprising the following steps:
[0025] Mix p-halogen nitrobenzene, N,N-dimethyl-p-phenylenediamine, a basic catalyst and a first polar solvent, and perform a first substitution reaction to obtain a diphenylamine derivative with a structure shown in formula II;
[0026] Mixing the diphenylamine derivative, the brominated fluorophore compound, the copper catalyst, the cocatalyst, the phase transfer catalyst and the second polar solvent, and performing a coupling reaction to obtain a mononitro compound with the structure shown in formula III;
[0027] Mixing the mononitro compound, the palladium carbon catalyst, hydrazine hydrate and the first mixed solvent, and performing the first reduction reaction to obtain the monoamino compound of the structure shown in formula IV;
[0028] Mixing the monoamino compound, p-halogen nitrobenzen...
Embodiment 1
[0086] Preparation of 4-N,N-dimethyl-4'nitrodiphenylamine:
[0087] Under nitrogen protection, add 27.2g (200mmol) N,N-dimethyl-p-phenylenediamine, 25.4g (180mmol) p-fluoronitrobenzene and 18.2g (180mmol) triethylamine into a 500mL three-necked flask, add 200mL DMSO, the solid content of the mixed system is 24.3wt%, reacted at 85°C for 20 hours, discharged in ice water, dried to obtain a dark red solid crude material, the crude material was mixed with ethanol and DMF with a volume ratio of 4:1 Liquid recrystallization, obtains purple crystal 42g, productive rate is 91%, structural formula is:
[0088]
[0089] Under nitrogen protection, 14.9g (58.0mmol) of the 4-N,N-dimethyl-4'nitrodiphenylamine, 10.0g (48.3mmol) of 2-bromonaphthalene, 46.0g (241.5mmol) of iodide Cuprous, 69.2g (193.2mmol) cesium carbonate and 12.2g (33.8mmol) dibenzo-18-crown-6 were added in a 500mL three-necked flask, and 227mL dichlorotoluene was added. The solid content of the mixed system was 35wt%. ...
Embodiment 2
[0098] Under nitrogen protection, 10.4g (40.5mmol) of 4-N,N-dimethyl-4'nitrodiphenylamine prepared in Example 1, 8.0g (33.7mmol) of 2-bromo-6methoxynaphthalene, 10.7 g (168.5mmol) of copper powder, 18.6g (134.8mmol) of potassium carbonate and 5.3g (20.2mmol) of 18-crown-6 were added to a 250mL three-necked flask, and 75.0mL of dichlorotoluene was added to determine the solid content of the mixed system 36wt%, reacted at 175°C for 20h, filtered while hot to obtain the filtrate, distilled off the dichlorotoluene solution under reduced pressure, and carried out column layering of the solid crude material with a dichloromethane / petroleum ether developer with a volume ratio of 2.5:1 Analyze and purify, obtain 9.3g red mononitro compound 4-nitrophenyl-4-N, N dimethylphenyl-2-amino-2-bromo-6-methoxynaphthalene, productive rate is 56.0% , with the following structure:
[0099]
[0100] Under nitrogen protection, in a 250mL three-necked flask, 8.0g (19.3mmol) of the above-prepared ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com