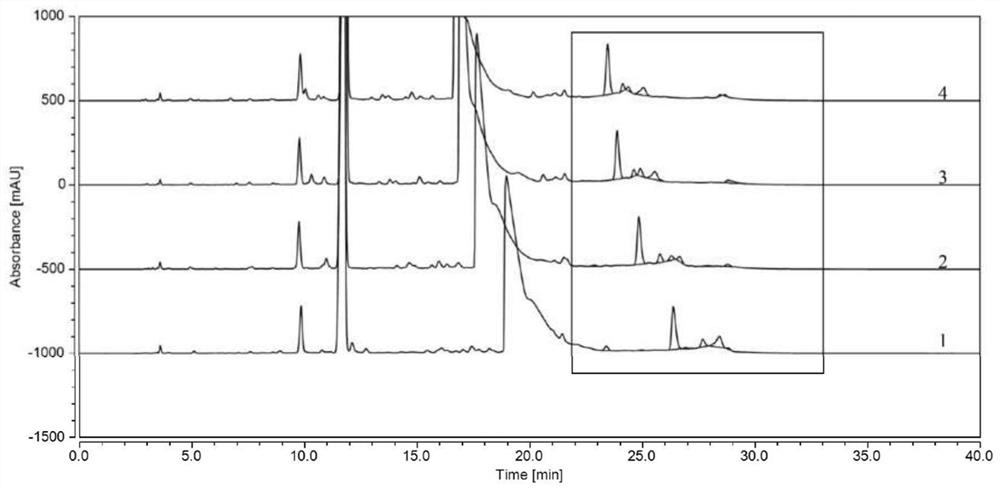
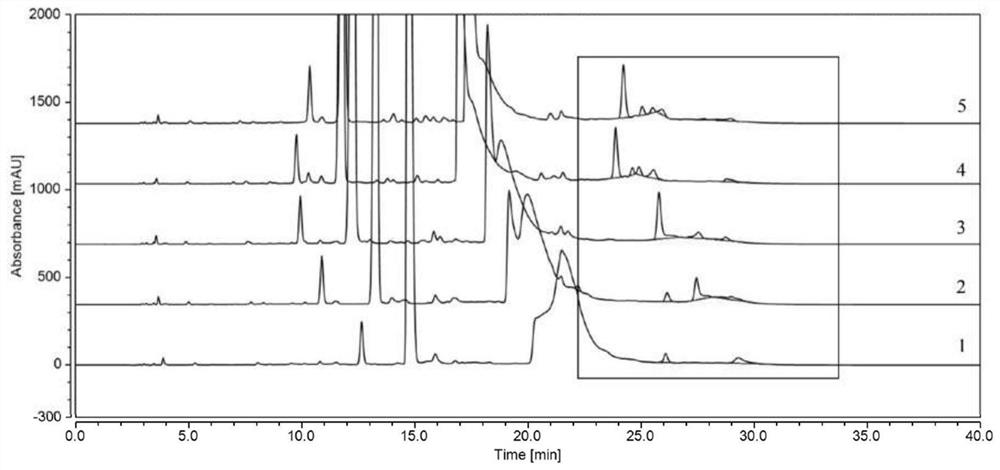
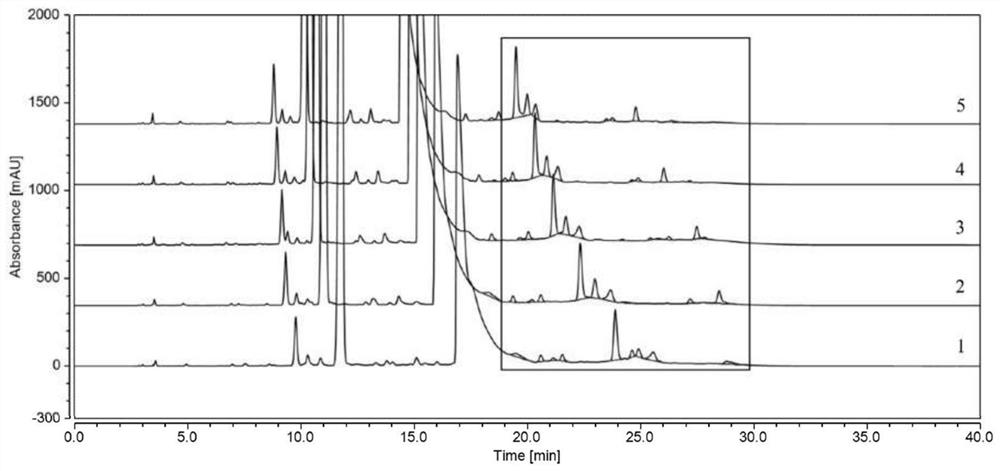
Method for detecting polymer impurities in cefadroxil raw material medicine and preparation of cefadroxil raw material medicine
A technique for detecting cefadroxil and its detection method, which is applied in the field of detection of polymer impurities in cefadroxil bulk drug and its preparations, can solve problems such as poor method specificity and small molecule impurity interference, and achieve good separation, good durability, good effect of specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] Preparation of polymer impurity localization control solution:
[0072] Weigh 40mg of cefadroxil raw material, put it in a vial, add 5ml of chloroform, then add 4 drops of triethylamine, shake well, dissolve into a solution with a concentration of 8mg / mL, and let it stand at 30±2°C in the dark. After about 36 hours, evaporate the solvent, dry in vacuum at 60±2°C for 30 minutes, add 5ml of water to dissolve, freeze-dry to solid powder, seal and store away from light, and obtain the cefadroxil polymer impurity positioning reserve sample;
[0073] Accurately weigh an appropriate amount of the cefadroxil polymer impurity localization reserve sample, add mobile phase A and quantitatively dilute it into a solution with a concentration of 5 mg / ml to obtain the polymer impurity localization control solution;
[0074] Preparation of the test solution:
[0075]Accurately weigh the cefadroxil bulk drug, add mobile phase A to dissolve and quantitatively dilute to a solution contai...
Embodiment 1
[0098] Example 1 Establishment of detection method for polymer impurities in cefadroxil bulk drug
[0099] The polymer impurity positioning control solution used in this embodiment was prepared by the following method:
[0100] Weigh 40 mg of cefadroxil raw material, put it in a vial, add 5 ml of chloroform, then add 4 drops of triethylamine, shake well, dissolve into a solution with a concentration of 8.0 mg / mL, and store it at 30±2°C in the dark. Set aside for about 36 hours, evaporate the solvent, dry in vacuum at 60±2°C for 30 minutes, add 5ml of water to dissolve, freeze-dry to solid powder, seal and store away from light, and obtain the cefadroxil polymer impurity positioning reserve sample;
[0101] Precisely weigh an appropriate amount of the cefadroxil polymer impurity localization reserve sample, add mobile phase A and quantitatively dilute to a solution with a concentration of 5 mg / ml to obtain the polymer impurity localization control solution.
[0102] The test...
Embodiment 2
[0165] Example 2 Isolation and structure identification of cefadroxil polymer impurity
[0166] In the process of research and establishment of polymer impurity detection methods, it is a key step in the research to obtain a polymer impurity reference substance with a clear structure and to effectively separate and identify the structure of polymer impurities.
[0167] In this example, the column switching LC / MSn method (C18-SW-C18) was used to separate and identify the polymer impurities in the polymer impurity control solution.
[0168] One-dimensional chromatographic system: Example 1 is optimized and established for the separation of polymer impurities.
[0169] Two-dimensional chromatography system: used for desalting and MS analysis of target impurities.
[0170] Mobile phase: IIA phase: formic acid-water (0.5:100, v / v), IIB phase: formic acid-acetonitrile (0.5:100, v / v), gradient elution, see Table 6, flow rate: 0.7ml / min.
[0171] Two-dimensional chromatographic co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com