Sinus stent and system and method of deploying stent within sinus of patient
A frontal sinus cavity and body part technology, applied in stents, devices for human tubular structures, medical science, etc., can solve the problems of reducing restenosis rate, lack of frontal sinus patency and mucosal recovery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
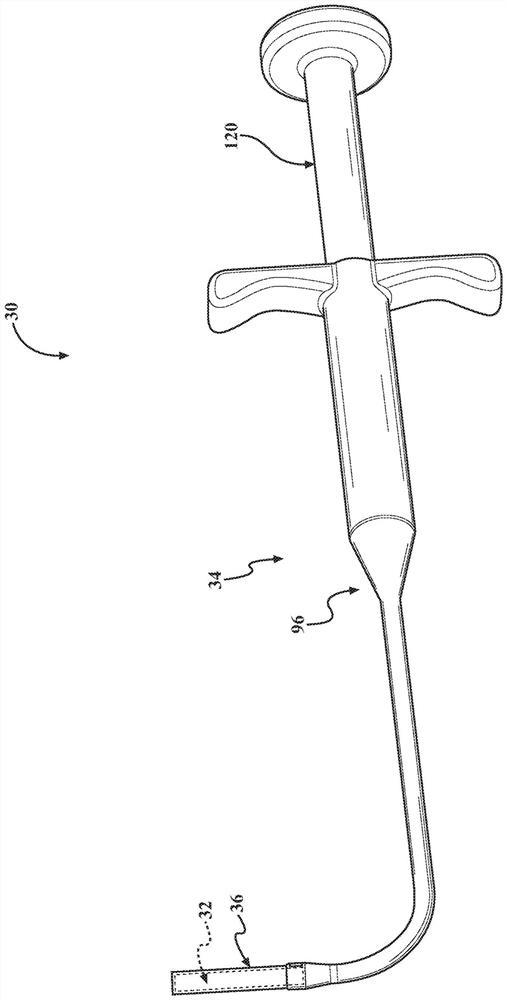
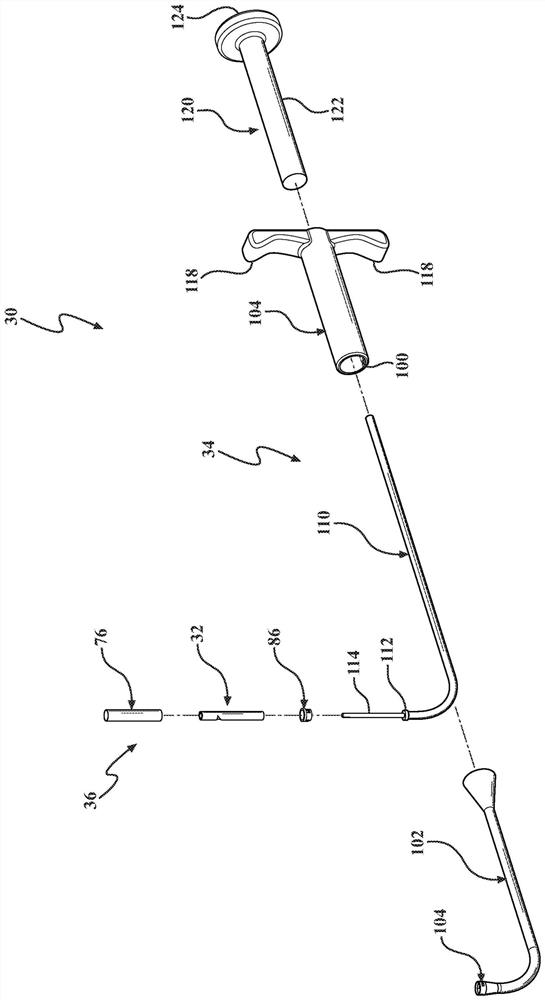
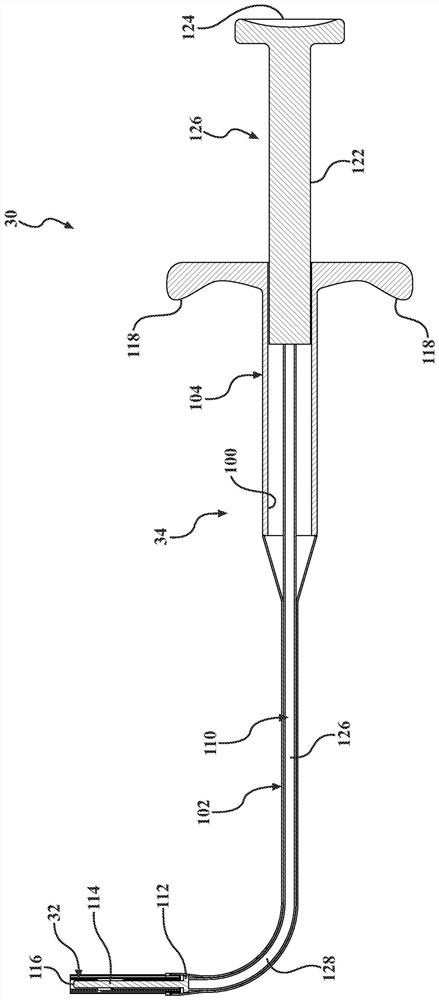
[0043] Figure 1-3 The frontal sinus (FS) for deploying the stent 32 to the patient is shown (see Figure 17 ) in the system 30. System 30 addresses the difficult visualization and anatomically complex challenges often associated with frontal sinus surgery. System 30 may be particularly suited for functional endoscopic (nasal) sinus surgery (FESS), a minimally invasive surgical technique that opens sinus air cells and sinus ostia with direct visualization. In one example, stent 32 is deployed in the frontal sinus after FESS to reduce, minimize and / or eliminate restenosis of the frontal sinus opening (FSO) (i.e., ostium), which is associated with chronic (rhino)sinusitis (CRS ) related to one of the main postoperative indicators of long-term outcome. System 30 can also be used to treat conditions such as inflammation, mucosal swelling, polyposis, and adhesions, thereby reducing recurrence of symptoms and / or the need for further surgical intervention. In addition, in a manne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com