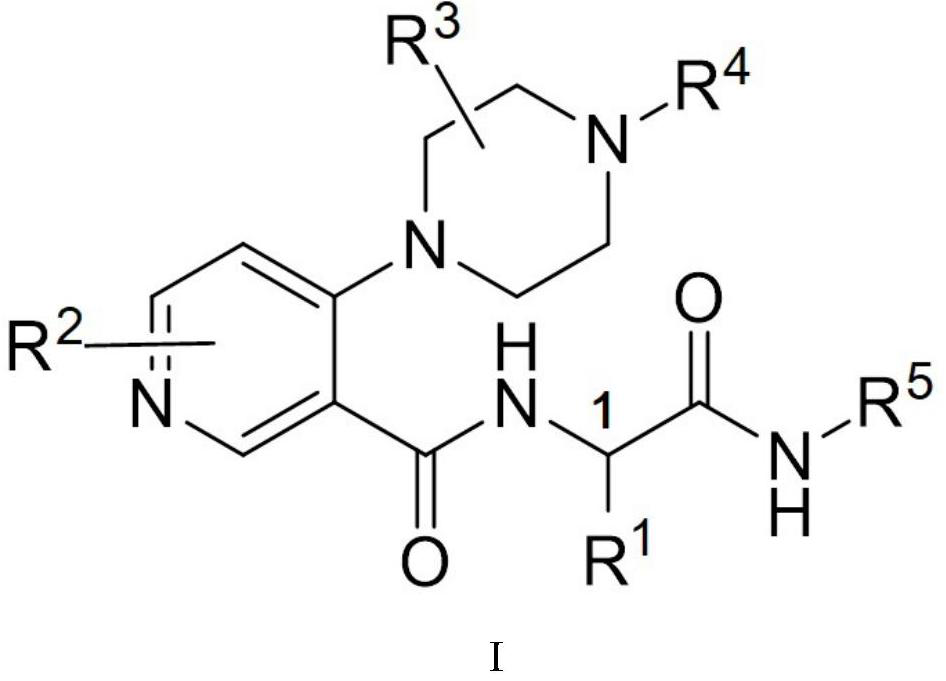
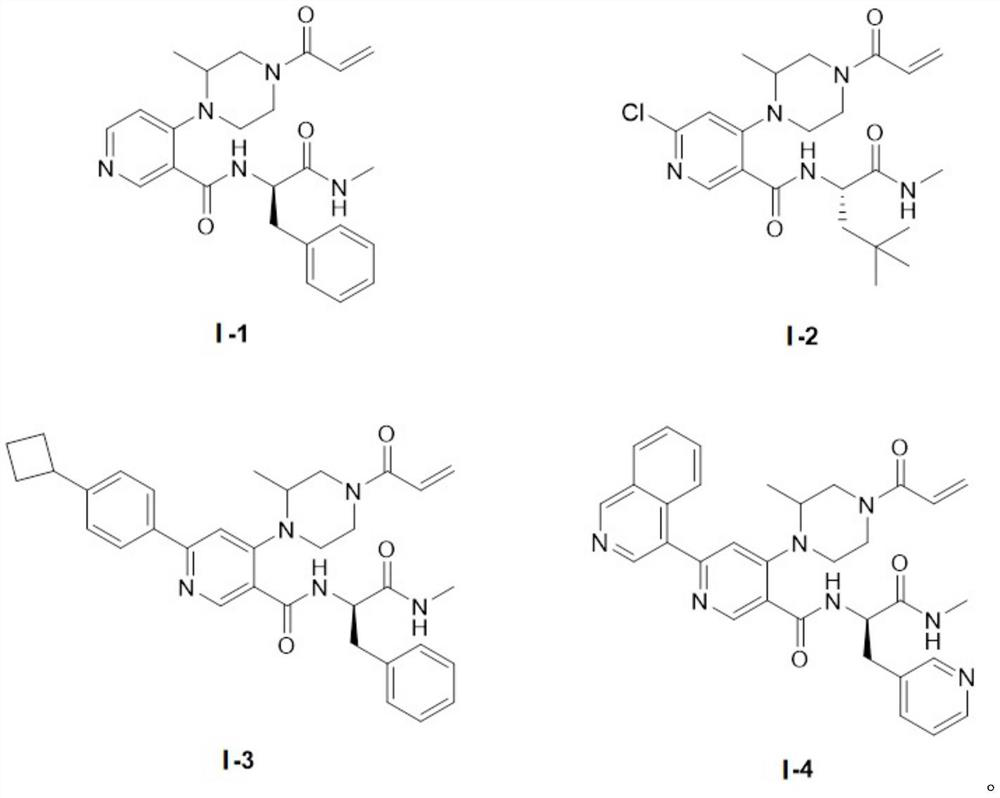
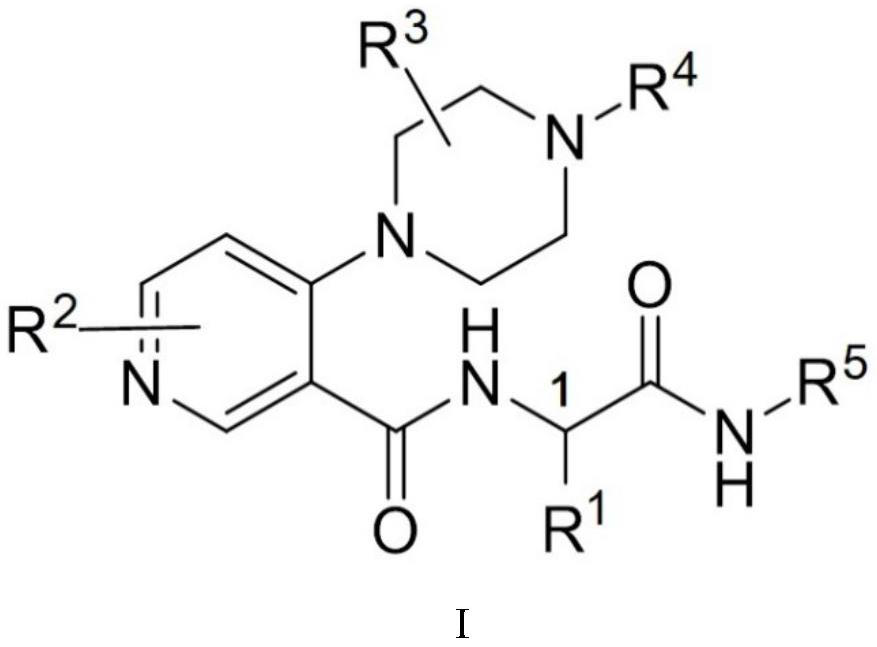
Piperazine compound and application thereof
A compound, piperazine technology, applied in the field of piperazine compound and its application, can solve problems such as shock, achieve low production cost, simple preparation process, and excellent inhibitory activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 4-(4-acryloyl-2-methylpiperazin-1-yl)-N-((R)-1-(methylamino)-1-oxyylidene-3-phenylpropan-2-yl ) Synthesis of nicotinamide (I-1)
[0047]
[0048] Step 1. Synthesis of 4-(2-chloro-5-methoxycarbonyl-4-pyridyl)-3-methyl-piperazine-1-carboxylic acid tert-butyl ester (23)
[0049]
[0050] Add 20mL DMF solvent to the reaction flask, weigh compound 21 (4.00g, 19.4mmol) into the reaction flask, and then add K 2 CO 3 (8.05g, 58.3mmol), compound 22 (5.83g, 29.1mmol). The reaction was performed at 55° C., and whether the reaction was complete was checked by TLC. After the reaction, add H 2 O (10 mL) quenched the reaction. After the reaction was quenched, CH 2 Cl 2 (20mL×2) to extract, collect the organic phase solution, and use Na 2 SO 4 dry. Filter out Na 2 SO 4 Afterwards, the organic solvent was spin-dried. The product 23, (white liquid, 2.44 g, 6.44 mmol, 33.2% yield) was obtained by separation by column chromatography separation technique.
[0051] LC-MS:...
Embodiment 2
[0075] Research on the inhibitory activity of compounds on target proteins
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com