A method for detecting milobalin and its enantiomer impurities by high performance liquid chromatography
A high-performance liquid chromatography and enantiomer technology, applied in the field of pharmaceutical analysis, can solve the problems of weak ultraviolet absorption, limited normal-phase method development, large polarity, etc., and achieve the effects of good durability and good injection repeatability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
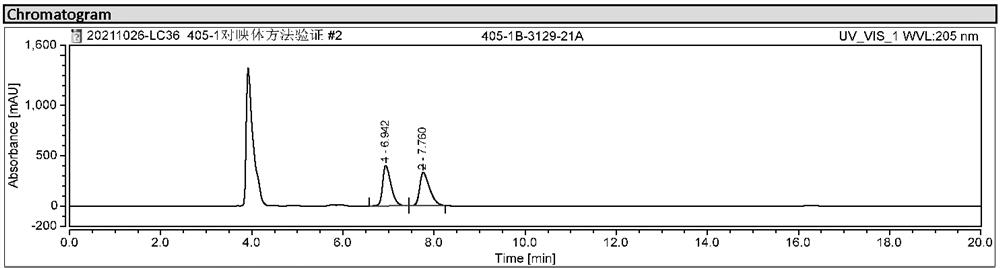
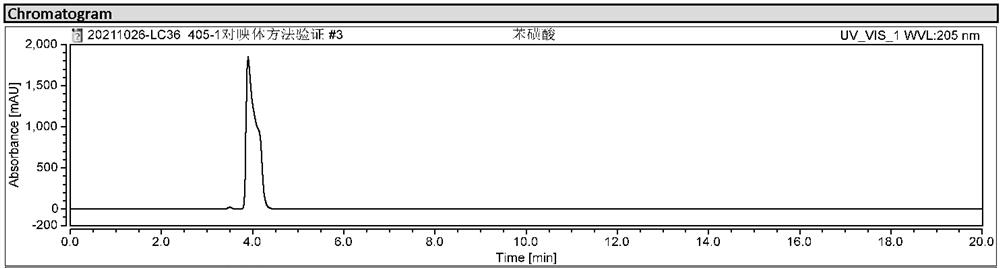
[0024] Embodiment 1: Screening of chromatographic conditions
[0025] Screen and determine the chromatographic conditions through chromatographic column, mobile phase, wavelength, flow rate, etc.
Embodiment 1-a
[0027] Chromatographic column: Daicel ADH 250×4.6mm, 5um;
[0028] Detection wavelength: 265 nm; column temperature: 25 ℃; injection volume: 10 uL; flow rate: 0.5 mL / min;
[0029] The mobile phase is n-hexane:ethanol=90:10, and the elution is isocratic according to 0 min and 60 min;
[0030] Conclusion: Using neutral system and Daicel ADH column, the peak elutes earlier and the peak shape is poor.
Embodiment 1-b
[0032] Try adding trifluoroacetic acid and diethylamine to the mobile phase on the basis of Example 1-a;
[0033] Chromatographic column: Daicel ADH 250×4.6mm, 5um;
[0034] Detection wavelength: 265 nm; column temperature: 25 ℃; injection volume: 10 uL; flow rate: 0.5 mL / min;
[0035] The mobile phase is n-hexane: ethanol: trifluoroacetic acid: diethylamine=90: 10: 0.05: 0.1;
[0036] According to 0 min, 60min isocratic elution;
[0037] Conclusion: Adding trifluoroacetic acid and diethylamine to the mobile phase on the basis of Example 1-a, the main component and enantiomers of milopalin came out earlier, and the peak shape was slightly improved, but they were not separated.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com