Method for separating milobalin intermediate and enantiomer thereof by high performance liquid chromatography
A high-performance liquid chromatography and enantiomer technology, which is applied in the field of high-performance liquid chromatography for separating milobaline intermediates and their enantiomers, and can solve the problems of cumbersome processing, low safety, problems such as low sensitivity, to achieve the effect of good injection repeatability, high safety and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Screening of Chromatography (Positive Phase)
[0035] The chromatographic conditions were determined by column, mobile phase, wavelength, flow rate, and the like.
Embodiment 1-a
[0037] Column: AD-H column 250mm × 4.6mm, 5 μm;
[0038] Test wavelength: 210 nm; column temperature: 30 ° C; injection volume: 10 μL; flow rate: 0.5 mL / min;
[0039] The flow phase is n-hexane: ethanol = 80:20, eluting according to 0 min, 40 min et al.;
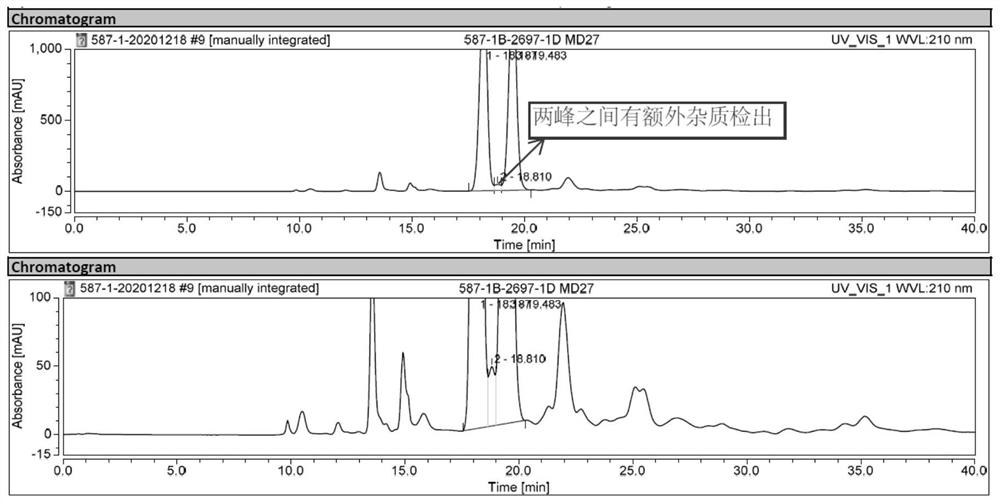
[0040] Conclusion: AD-H pillar, Milo Bahrain intermediate and its enantiomer peak peaks are premature, and the separation is poor.
Embodiment 1-b
[0042] Attempts to adjust the flow phase and the column temperature on the basis of Example 1-A;
[0043] Column: The AD-H column 250mm × 4.6mm, 5 μm;
[0044] Detection wavelength: 210 nm; column temperature: 25 ° C; injection volume: 10 μL; flow rate: 0.5 mL / min;
[0045] The flow phase is n-hexane: isopropanol = 98: 2, eluting according to 0 min, 45 min is equal;
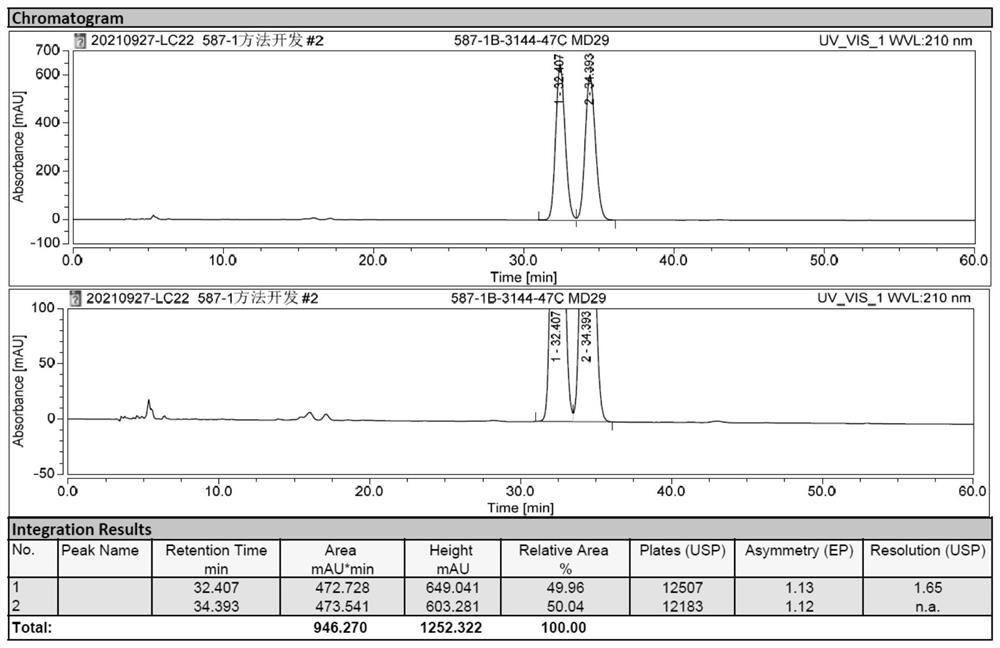
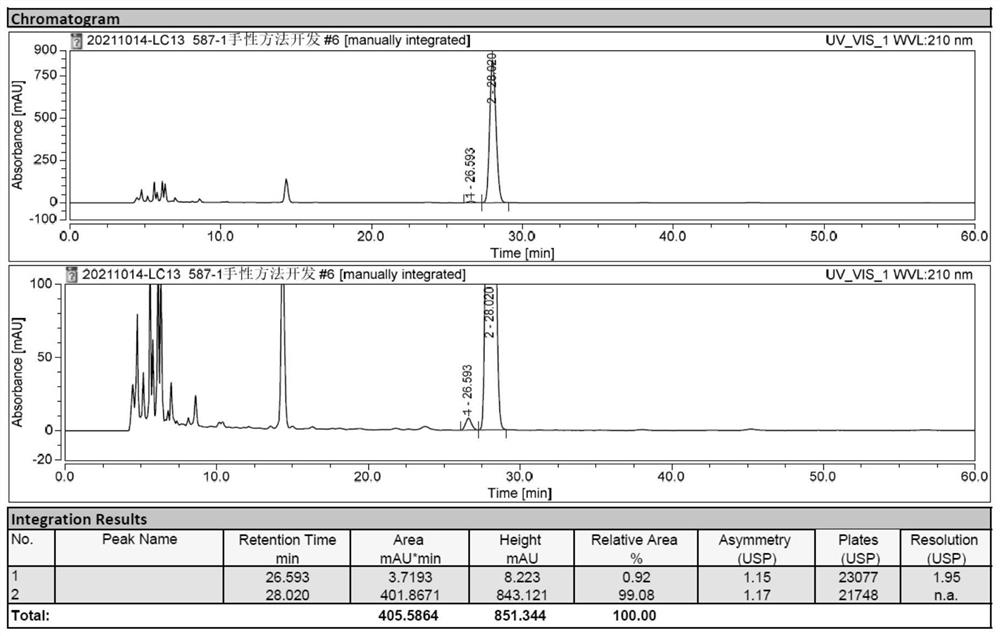
[0046] Conclusion: AD-H column is used to change the mobile phase and its ratio. After adjusting the column temperature, the Milo Bahrain Intermediate and its enantioma separation are slightly improved, but it has not yet reached the baseline separation requirements.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com