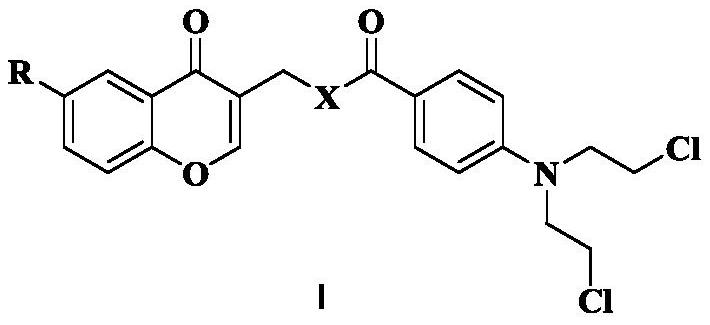
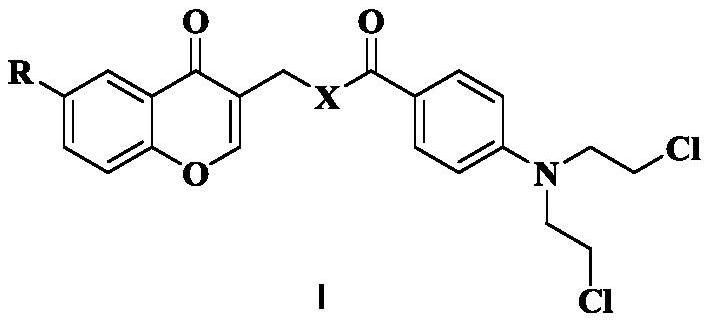
Chromone nitrogen mustard derivative and anti-tumor application
A technology for chromogen mustard and derivatives, which is applied in the fields of natural medicine and medicinal chemistry, can solve problems such as drug resistance with side effects, and achieve the effect of good anti-tumor cell proliferation effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
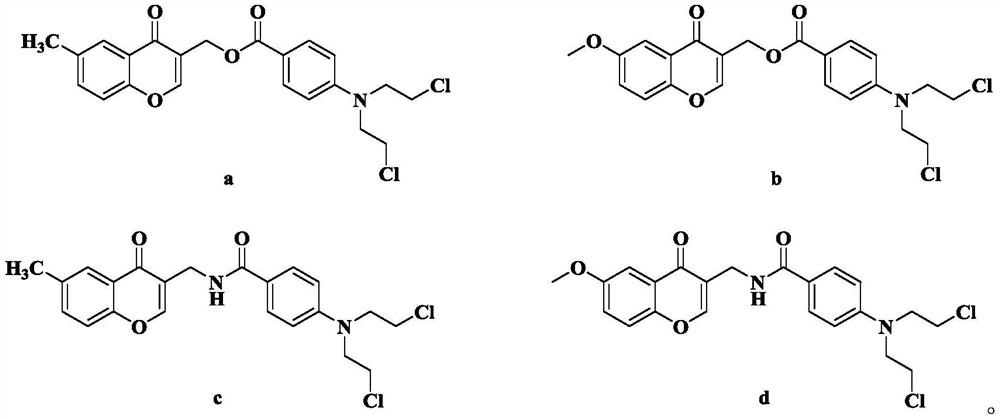
[0031] (1) Dissolve 500 mg of compound 1a (3.33 mmol) in 10 mL of DMF, then add 630 μL of phosphorus oxychloride (6.72 mmol) dropwise, and react at room temperature for 12 h. TLC monitoring showed that the reaction was almost complete. When 15mL of water was added, crystals were precipitated. After suction filtration and drying, 402.4mg of intermediate 2a was obtained. Dissolve 50 mg of intermediate 2a (0.27 mmol) in 10 mL of isopropanol and add 1 g of basic Al 2 o 3 (9.80mmol), and then reflux at 75°C for 5h. TLC monitoring, the reaction is complete, suction filtration, the filtrate is concentrated to obtain a crude product, which is separated by silica gel column chromatography (DCM:MeOH) to obtain compound 3a (R=-CH 3 ).
[0032] (2) Add 5 mL of ethylene oxide (0.10 mol) to a suspension of 1.53 g of ethyl 4-aminobenzoate 5 (9.26 mmol) dissolved in 12 mL of 65% aqueous acetic acid, and stir at room temperature for 24 h. After the reaction was complete, it was...
Embodiment 2
[0035]
[0036] The step of synthesizing 3a in step (1) of Example 1 was replaced by: dissolving 553 mg of compound 1b (3.33 mmol) in 10 mL of DMF, then adding 630 μL of phosphorus oxychloride (6.72 mmol) dropwise, and reacting at room temperature for 12 h. TLC monitoring showed that the reaction was almost complete. When 15mL of water was added, crystals were precipitated. After suction filtration and drying, 410mg of intermediate 2b was obtained. Dissolve 55 mg of intermediate 2b (0.27 mmol) in 10 mL of isopropanol and add 1 g of basic Al 2 o 3 (9.80mmol), then reflux at 75°C for 5h. Monitored by TLC, the reaction was complete, suction filtered, and the filtrate was concentrated to obtain a crude product, which was separated by silica gel column chromatography (DCM:MeOH) to obtain compound 3b (R=-OCH 3 ).
[0037] The rest of the steps were prepared according to the synthetic method of Example 1 to obtain compound 8b as a yellow oil with a yield of 43.8%. 1 HNMR (CDCl...
Embodiment 3
[0039]
[0040] The step of synthesizing 3a in step (1) of Example 1 was replaced by: dissolving 500 mg of compound 1a (3.33 mmol) in 10 mL of DMF, then adding 630 μL of phosphorus oxychloride (6.72 mmol) dropwise, and reacting at room temperature for 12 h. TLC monitoring showed that the reaction was almost complete. When 15mL of water was added, crystals were precipitated. After suction filtration and drying, 402.4mg of intermediate 2a was obtained. Dissolve 50 mg of intermediate 2a (0.27 mmol) in 10 mL of isopropanol and add 1 g of basic Al 2 o 3 (9.80mmol), and then reflux at 75°C for 5h. TLC monitoring showed that the reaction was complete. Suction filtration was performed, and the filtrate was concentrated to obtain a crude product, which was separated by silica gel column chromatography (DCM:MeOH) to obtain 15 mg of compound 3a. 500mg of compound 3a (2.62mmol) was dissolved in 10mL of DCM, 750μL of phosphorus tribromide (7.90mmol) was added dropwise under ice coolin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com