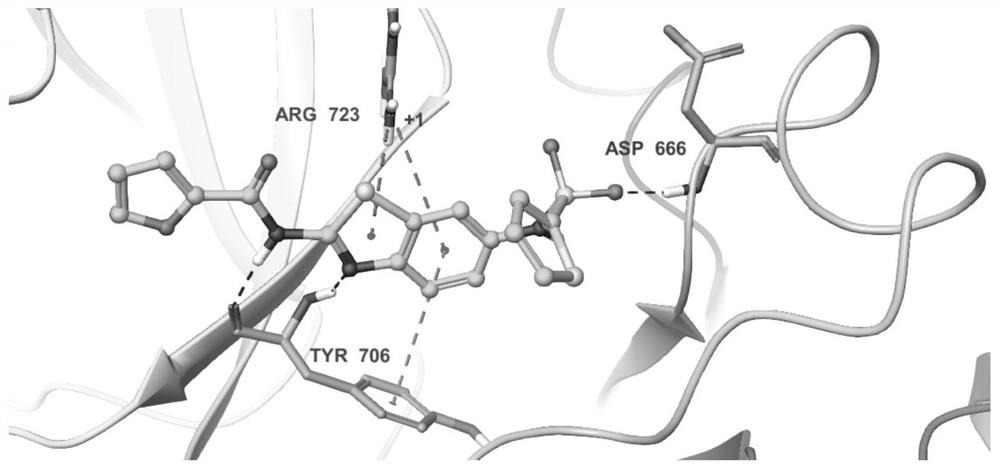
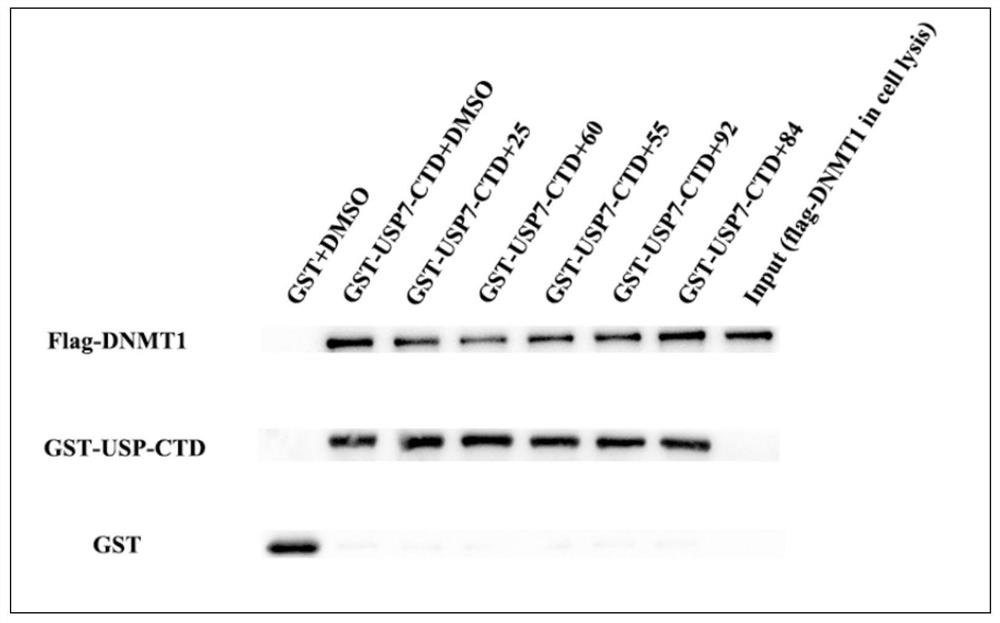
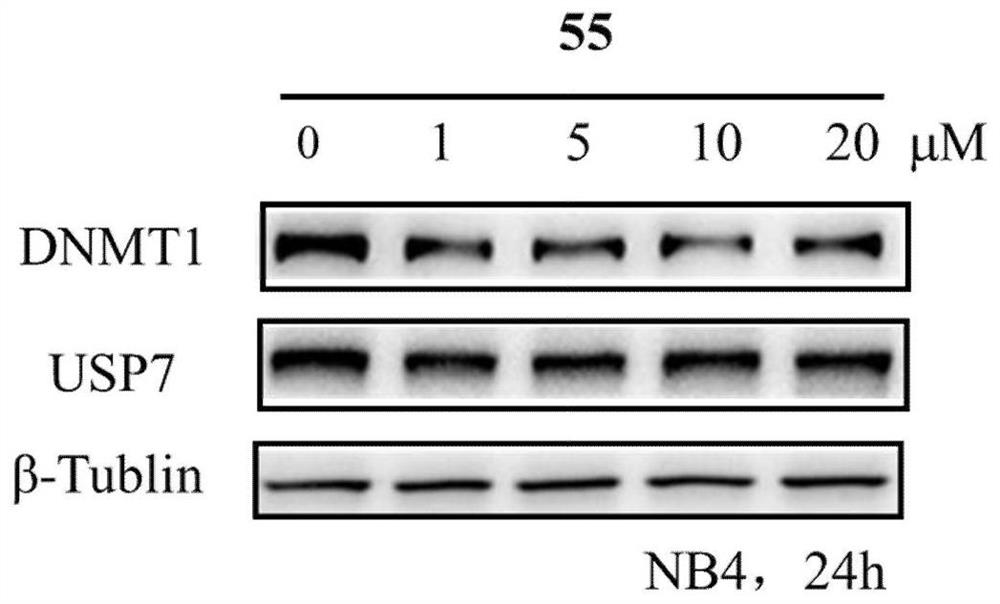
Benzothiazole compound and medical application
A technology of benzothiazole and compound, applied in the field of medicinal chemistry, can solve problems such as small toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] N-(2-(furan-2-carboxamide)benzo[d]thiazol-6-yl)isonicotinamide (Compound 1)
[0067]
[0068] Dissolve 2-amino-6-nitrobenzothiazole (1a, 1.0g, 5.13mmol) in pyridine (20mL), add fresh furoyl chloride (758μL, 7.70mmol) dropwise, heat up to 40°C and stir 8 hours. TLC monitored the completion of the reaction and added 1N hydrochloric acid (30 mL) for neutralization, and a large amount of solid precipitated out. After suction filtration, the solid was washed with a small amount of ethyl acetate (2 mL×3) to obtain the crude product of intermediate 1b, which was directly used in the next step without purification.
[0069] All the obtained crude intermediate 1b was added to methanol (25 mL), 10% palladium / carbon (100 mg) was added, hydrogen gas was introduced and stirred at 50° C. for 7 hours. TLC monitored that the reaction was complete and stopped heating. After cooling to room temperature, the solution was filtered with diatomaceous earth, and the filtrate was evaporated...
Embodiment 2
[0072] N-(2-(furan-2-carboxamide)benzo[d]thiazol-6-yl)pyridineamide (Compound 2)
[0073]
[0074] Referring to the method of Example 1, isonicotinic acid was replaced by picolinic acid to obtain compound 2 (off-white solid): 1 HNMR (300MHz, DMSO-d 6 )δ12.80(s,1H),10.80(s,1H),8.77(d,J=4.7Hz,1H),8.60(s,1H),8.20(d,J=7.9Hz,1H),8.15– 8.01(m,2H),7.95(d,J=8.9Hz,1H),7.84–7.60(m,3H),6.77(s,1H).ESI-MS m / z 363.1[M-H] - .
Embodiment 3
[0076] N-(2-(furan-2-carboxamide)benzo[d]thiazol-6-yl)pyrazine-2-carboxamide (compound 3)
[0077]
[0078] Referring to the method of Example 1, isonicotinic acid was replaced by pyrazine-2-carboxylic acid to obtain compound 3 (off-white solid): 1 H NMR (300MHz, DMSO-d 6 )δ12.83(s,1H),10.88(s,1H),9.33(s,1H),8.95(s,1H),8.83(s,1H),8.57(s,1H),8.04(s,1H ), 7.94(d, J=8.9Hz, 2H), 7.86–7.48(m, 2H), 6.76(s, 1H). ESI-MS m / z 364.1[M-H] - .
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| ion source temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com