Application of PCN (Pregnenolone-16a-carbonitrile) to preparation of medicine for treating related diseases of chronic kidney diseases
A technology for chronic kidney disease and related diseases, applied in the new application field of PCN, which can solve the problems of no research reports and no PCN yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
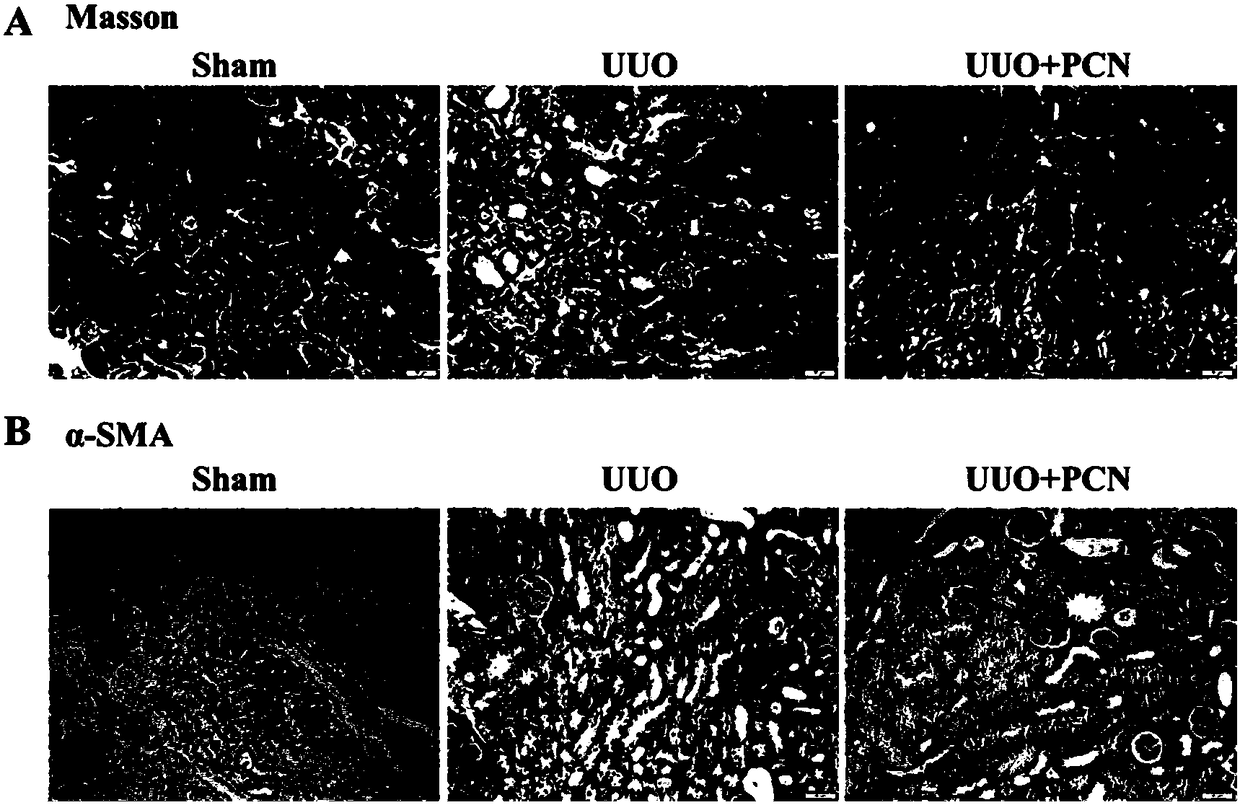
[0027] Example 1 Effect of PCN on UUO-induced CKD renal pathology.
[0028] Male C57BL / 6 mice weighing 18-22 g were taken and divided into 3 groups, namely the control group, the UUO model group and the PCN treatment group (n=8).
[0029] Control group: intraperitoneal injection of equal volume medium once a day for 8 days in total;
[0030] UUO model group: unilateral ureteral ligation;
[0031] PCN treatment group: PCN was administered for 2 days in advance (intraperitoneal injection, 100 mg / kg PCN, 200 μL / time), once a day, followed by unilateral ureteral ligation, followed by PCN treatment for 6 days after operation, and 6 days after unilateral ureteral ligation, they were executed Mice, blood and kidney tissue were collected.
[0032] The kidney samples of each group were made into wax blocks, sectioned and subjected to Masson staining to detect the degree of tubulointerstitial fibrosis. The experimental results are shown in figure 1 a. It can be seen from the results...
Embodiment 2
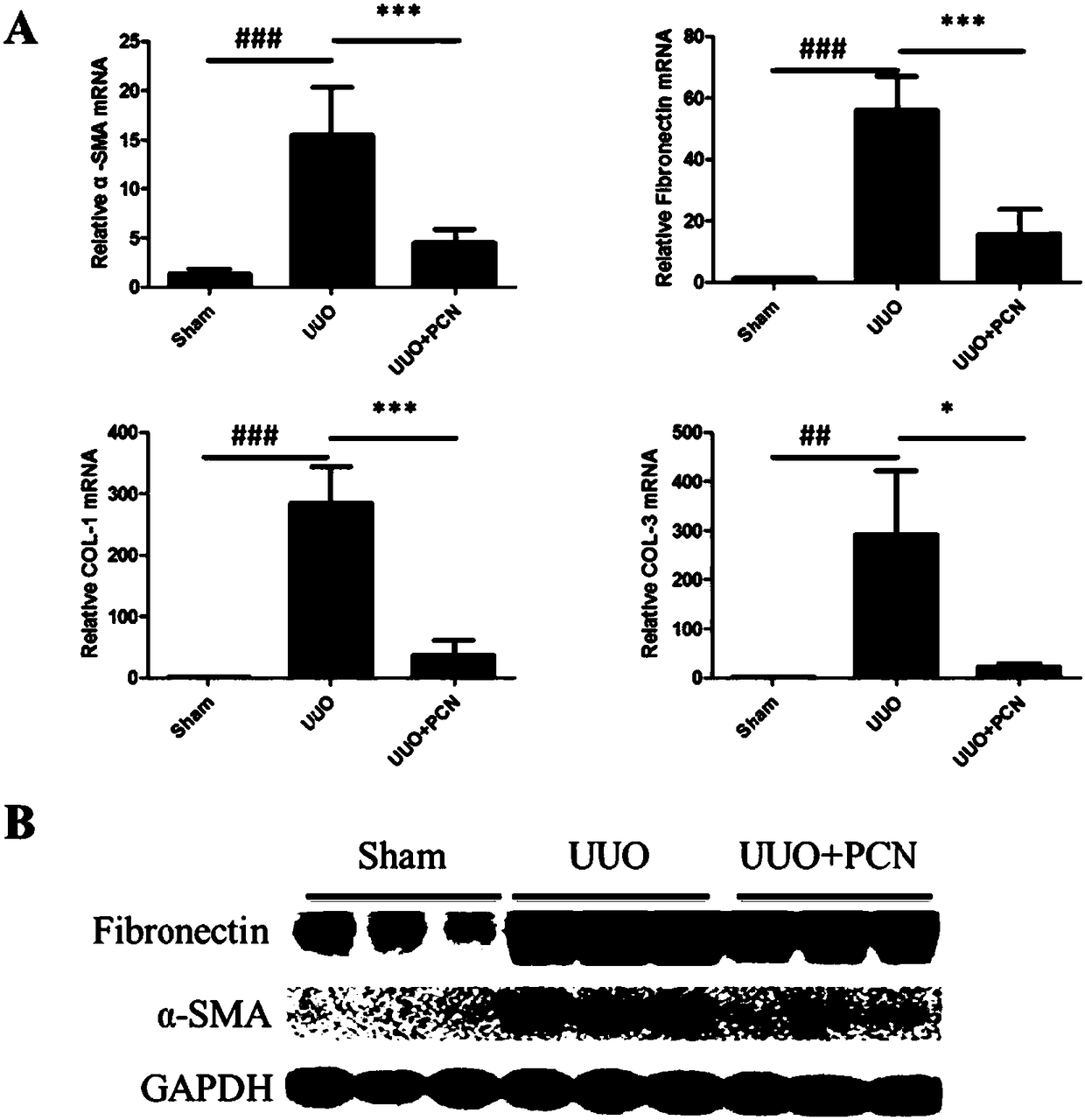
[0034] Example 2 The effect of PCN on the expression of molecules related to tubulointerstitial fibrosis induced by UUO.
[0035] The effect of PCN on the expression of UUO-induced tubulointerstitial fibrosis-related molecules was studied by QPCR and Western blot.
[0036] Such as figure 2 As shown in A, the effect of PCN on the expression of molecules related to tubulointerstitial fibrosis induced by UUO was studied by QPCR method. In the UUO-induced CKD model, the expression levels of fibrosis indicators α-SMA, Fibronectin, Collagen I, and Collagen III in the UUO model group were significantly higher than those in the control group, p<0.05. The PCN treatment group can significantly reduce the mRNA levels of α-SMA, Fibronectin, Collagen I, and Collagen III, p<0.01.
[0037] Such as figure 2As shown in B, the effect of PCN on the expression of molecules related to tubulointerstitial fibrosis induced by UUO was studied by Western blot. In the UUO-induced CKD model, the fi...
Embodiment 3
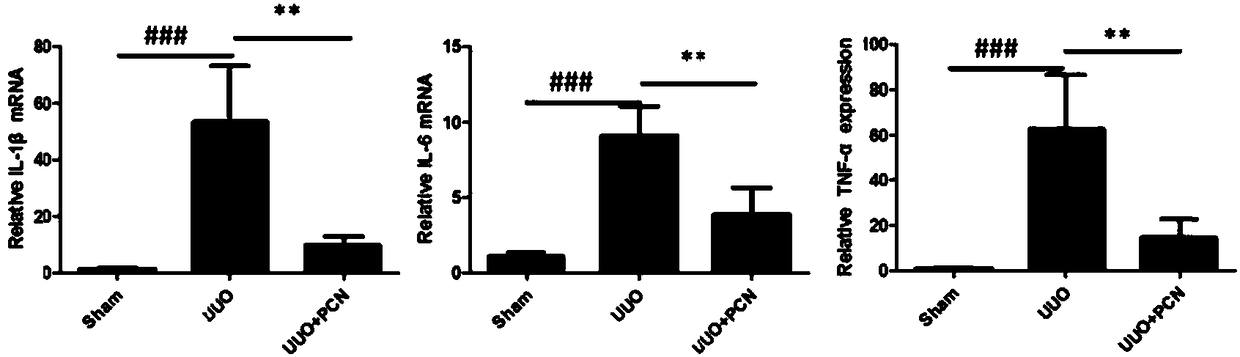
[0039] Example 3 Effect of PCN on UUO-induced renal inflammation-related molecules.
[0040] The expression levels of renal inflammation-related molecules were detected by QPCR. The result is as image 3 As shown, the expressions of inflammation-related molecules IL-1β, IL-6, and TNF-α in the UUO model group were significantly increased, p<0.001. The PCN treatment group can significantly reduce the expression levels of IL-1β, IL-6, TNF-α, p<0.05.
[0041] The results showed that PCN could significantly reduce the expression levels of UUO-induced renal inflammation-related molecules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com