Apoptosis-Inducing Agent for Prostate Cancer Cells
a prostate cancer and apoptosis-inducing technology, which is applied in the direction of peptides, drug compositions, peptides, etc., can solve the problems that most of their functions have not yet been elucidated, and achieve the effects of inhibiting prostate cancer, and reducing the expression level of reic/dkk-3 gen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
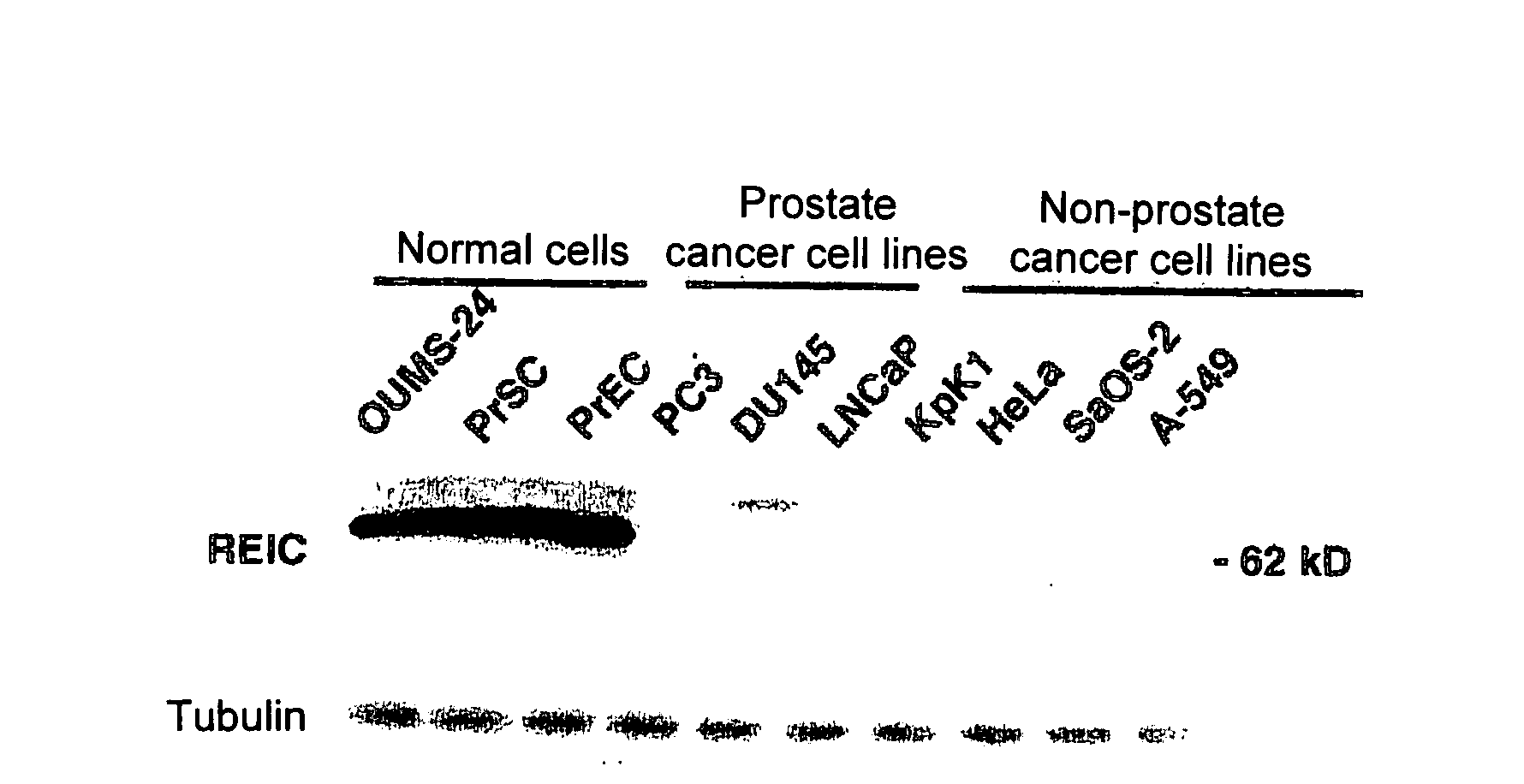
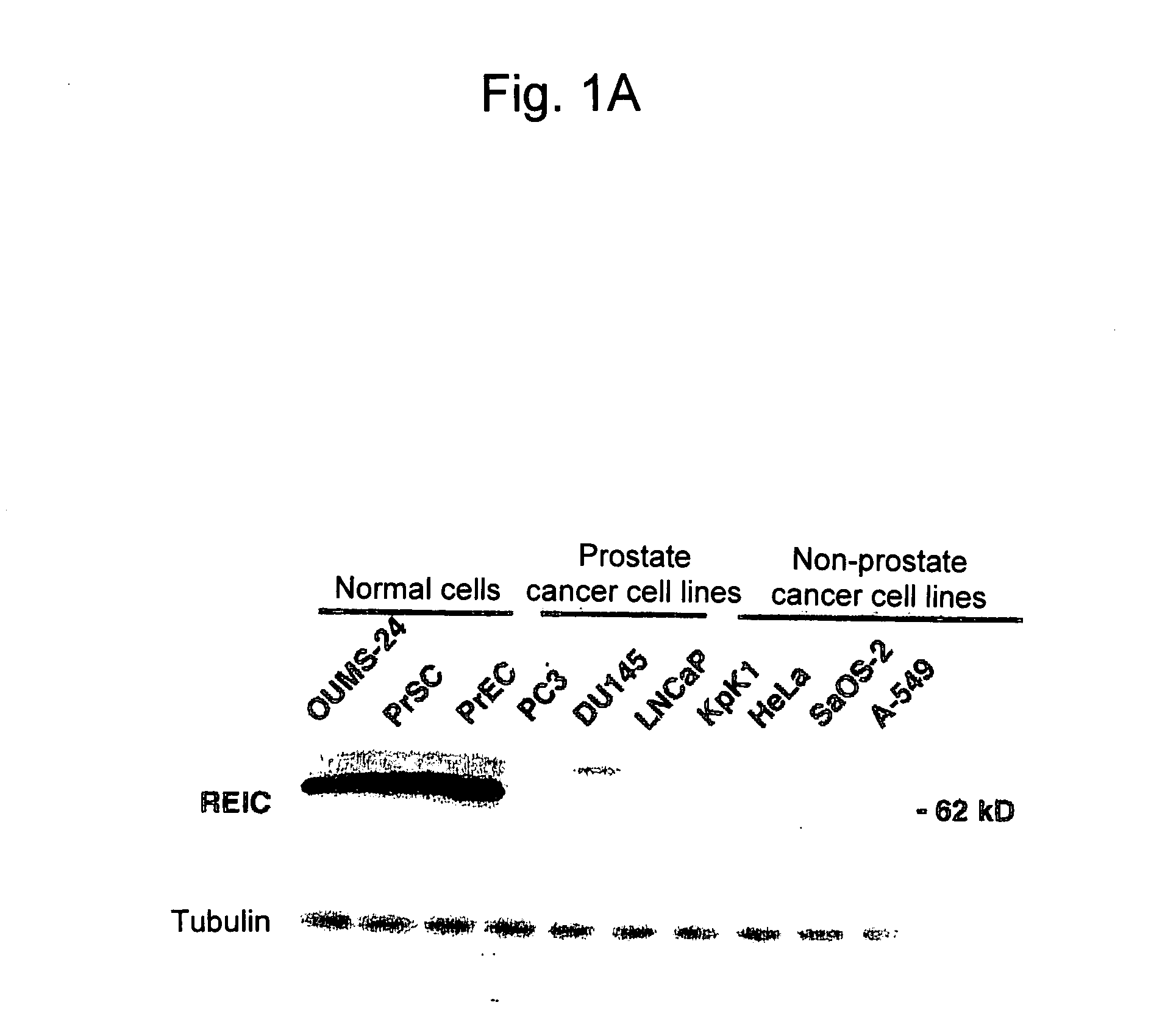
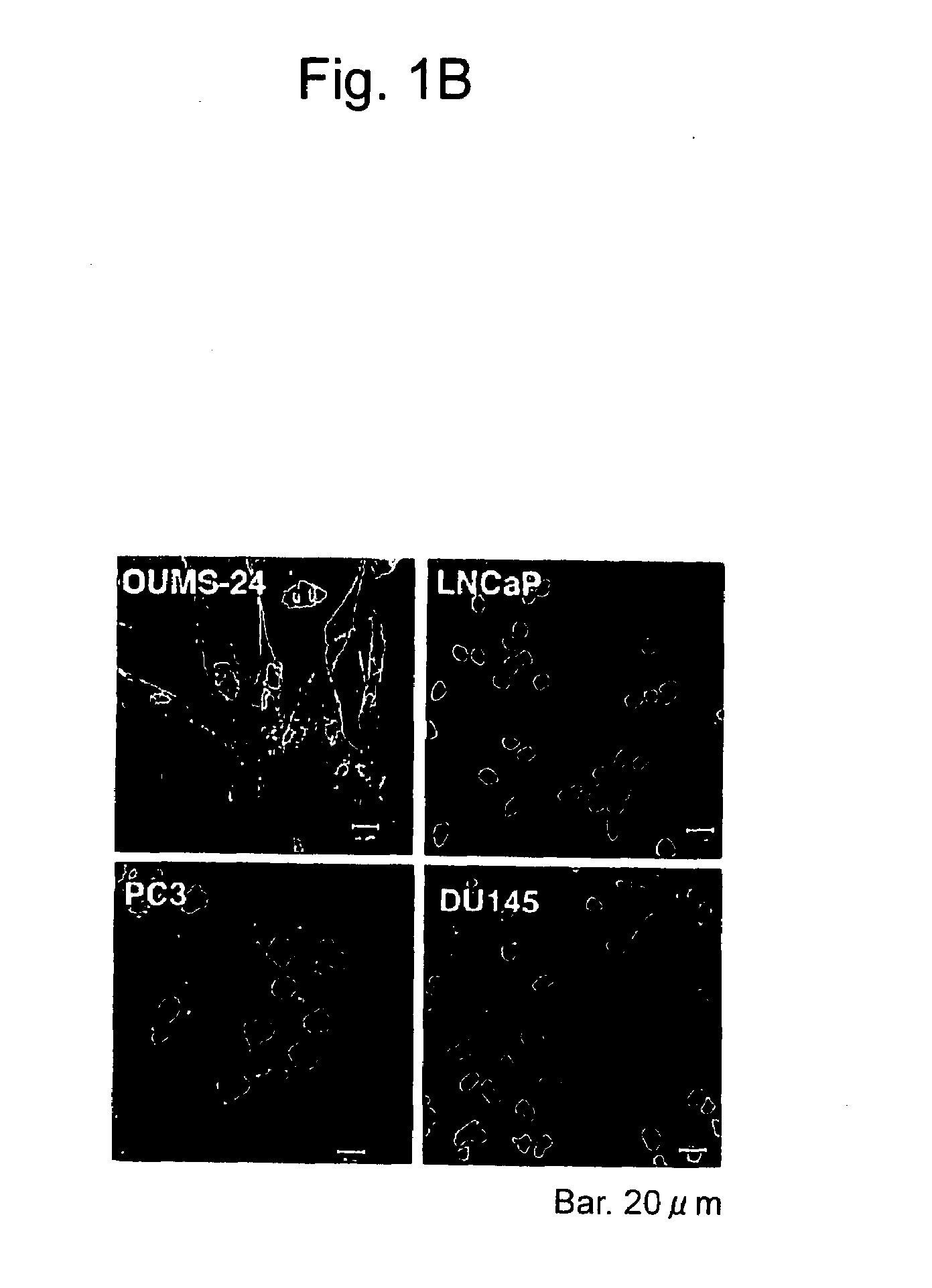
[0085]Prostate Cancer Cell Apoptosis Induction by REIC / Dkk-3
[0086]Materials used in the Examples of the present invention were obtained as described below. The Examples were carried out by methods described below.
Cells and Culture Method
[0087]Normal prostate epithelial cells (PrEC) and prostate stromal cells (PrSC) were purchased from Cambrex (Baltimore, Md.). The prostate cancer cell lines PC3, DU145, and LNCaP were provided by ATCC (Rockville, Md.). OUMS-24 was provided by Dr. Masayoshi Nanba. An HAM'S F-12 K medium, an RPMI 1640 medium, and modified MEM medium (Nissui) were separately used with a supplement of 10% calf serum for PC3, DU145 and LNCaP, and OUMS-24.
Human Prostate Tissue
[0088]A LandMark™ low-density prostate tissue microarray (Ambion, Austin) was used for immunostaining of the REIC / Dkk-3 gene. Fresh prostate cancer tissue samples were obtained from 40 patients. Of them, 20 samples had a Gleason score of 8 or higher and another 20 samples had a Gleason score of 7 or l...
example 2
[0105]Effects of Prostate Cancer Metastasis Inhibition caused by REIC / Dkk-3 Gene Transfection using Adenovirus Vectors
[0106]Mouse prostate cancer cells RM-9 (5.0×103 cells) were injected into prostates of C57BL / 6 mice. One week later, 1.2×108 pfu of REIC or lacZ (or PBS) was directly injected intratumorally (mean tumor diameter: 60 mm3) with the use of adenovectors. Tumor growth was significantly inhibited in the REIC / Dkk-3 group (treatment group) compared with the control group and the lacZ group (FIGS. 5 and 6). Tumor diameters were measured every 3 days using a transrectal ultrasonography system previously developed by the present inventors. Tumor volumes (V) were calculated by the following formula: ½×(short diameter)2×(long diameter). FIG. 5 shows ultrasound images and images of mouse intraperitoneal cavities taken with the transrectal ultrasonography system. FIG. 6 shows time-dependent changes in tumor volumes. As shown in FIGS. 5 and 6, stronger tumor inhibition effects were ...
example 3
[0112]Relationship between HSP70 and Apoptosis Induction caused by Forced Expression of REIC / Dkk-3
[0113]It was examined whether tumor selectivity in terms of apoptosis expression caused by Ad-REIC would be associated with HSP70 expression in normal cells and cancer cells.
[0114]Normal cells OUMS 24 (human immortalized fibroblast) and prostate cancer cells PC3 were used for comparison. The cells were infected with Ad-REIC at 20 MOI. 24 hours later, an HSP70 inhibitor (heat shock protein inhibitor I, CALBIOCHEM, BIOCHEMICALS) and an HSP70 inducer (Geranyl-Geranyl Acetone) (250 mM each) were separately added to a culture solution. 48 hours later, TUNEL staining was performed to determine influence upon apoptosis. FIGS. 12 and 13 show staining images for OUMS24 and of PC3, respectively. In FIGS. 12 and 13, images on the left side indicate TUNEL-staining results and images on the right side indicate DAPI-staining results. As shown in FIG. 12, among normal cells OUMS24, a large number of T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com