Synthesis method of novel urushiol hydroxamic acid derivatives with HDAC inhibition and antitumor activity
A technology of urushiol-based hydroxamic acid and anti-tumor activity, which is applied in the field of pharmaceutical synthetic chemistry and can solve the problem of lack of zinc ion binding region, a key structural unit of HDAC inhibition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
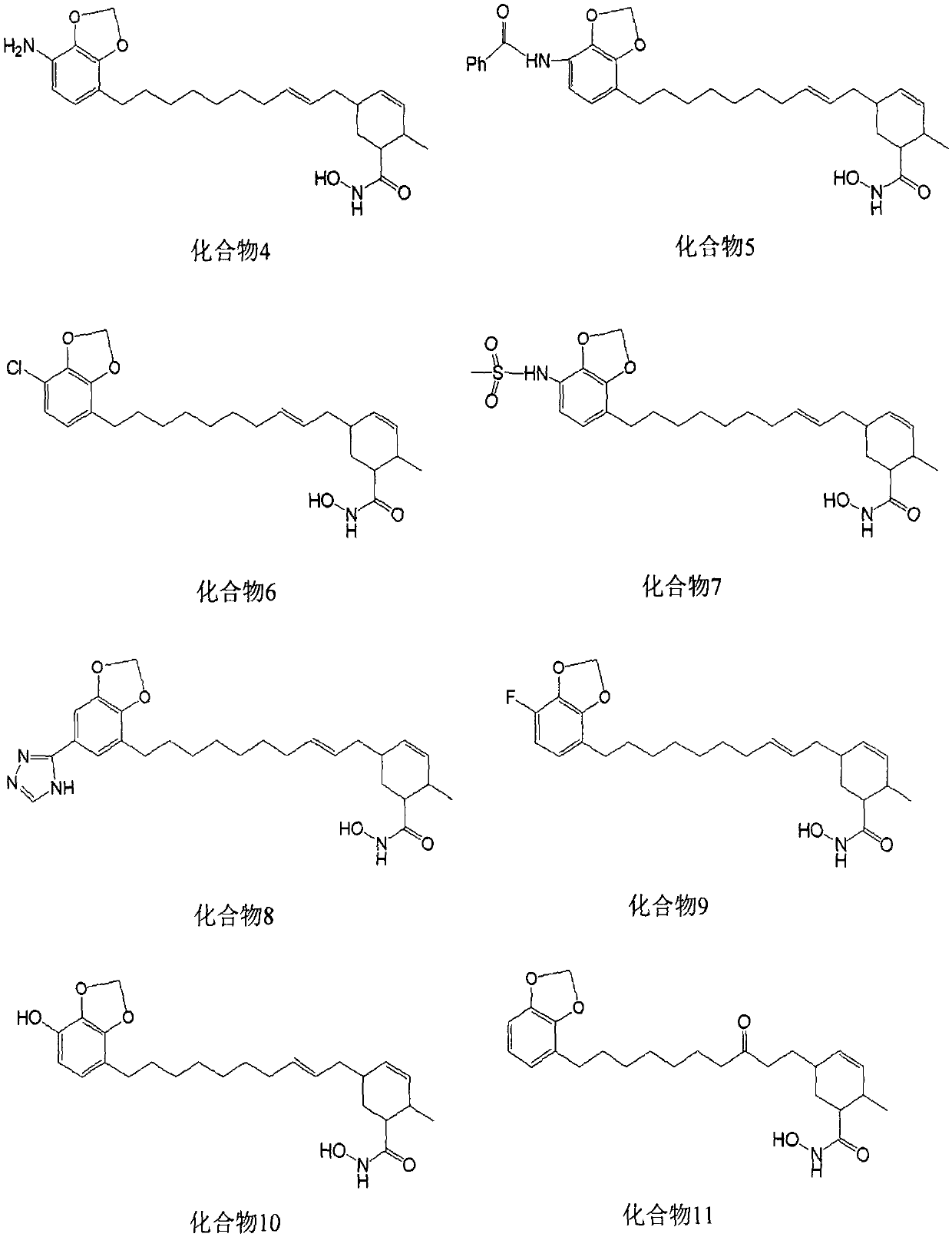
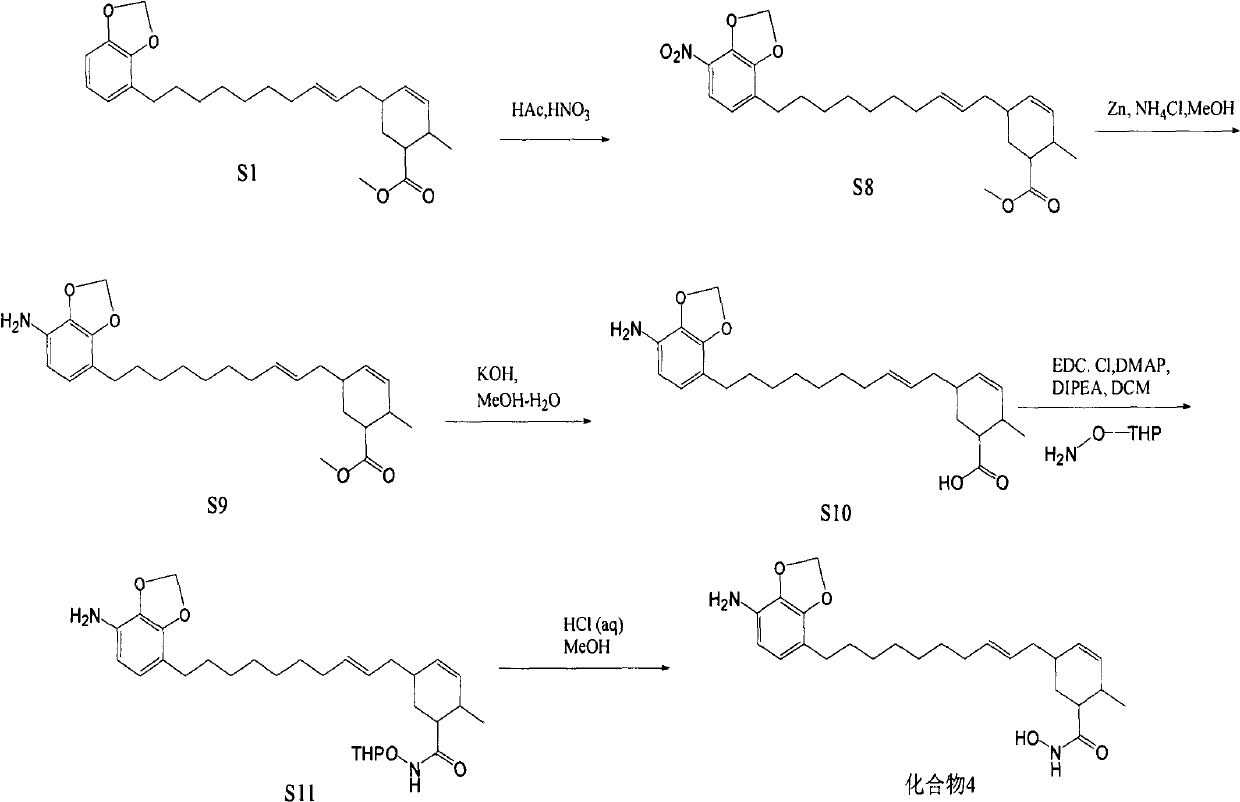
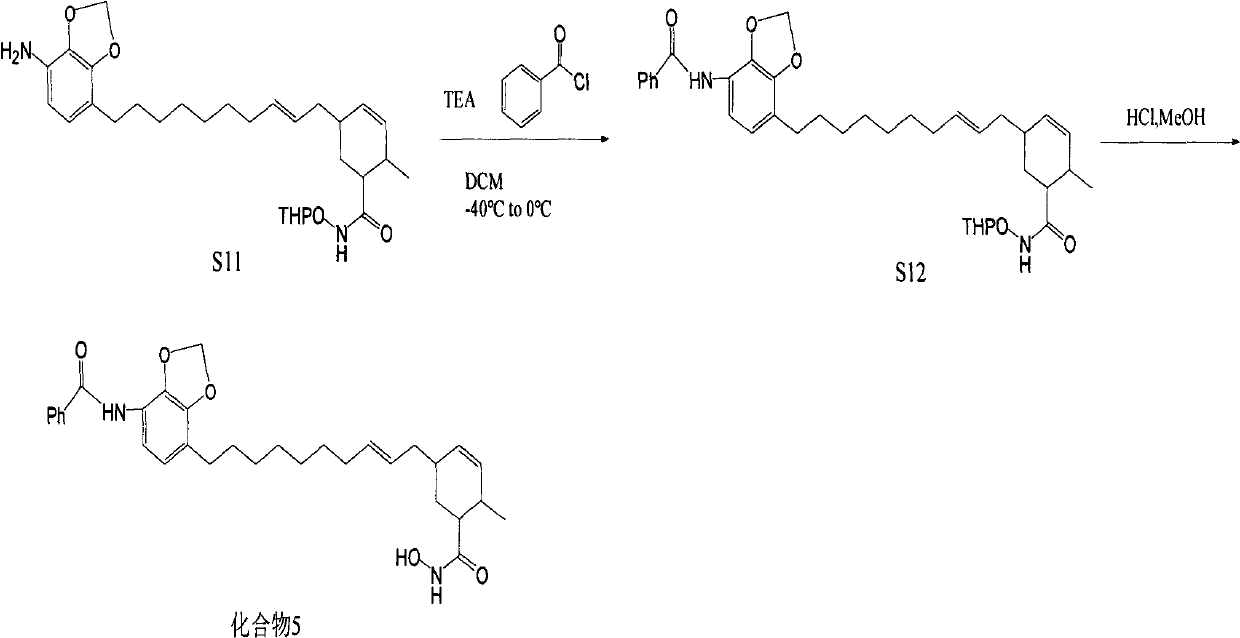
[0060] Synthesis of target compound 4
[0061] Dissolve 5.5g of compound S1 in 50mL of acetic acid, cool down to 0°C, add 20mL of concentrated nitric acid under vigorous stirring, add 100mL of water after 6 hours, extract 4 times with ethyl acetate, each time 100mL, combine the organic layers with saturated carbonic acid Wash twice with sodium hydrogen solution, 200 mL each time, and dry with anhydrous magnesium sulfate; evaporate the solvent to dryness under reduced pressure to obtain a light yellow oil, take 2.3 g of it and dissolve it in 100 mL of methanol, put it in an ice-water bath, and add 6.0 g of anhydrous ammonium chloride , slowly add 8.0g of zinc powder, add 100mL of EA and 600mL of PE after reacting for 20min, filter, evaporate the filtrate to dryness, and quickly carry out silica gel column chromatography, elute with PE-EA, PE:EA=5:1, wash Remove the liquid and evaporate the solvent to dryness under reduced pressure to obtain 0.6g of brown oil, dissolve all of it...
Embodiment 2
[0077] Structural identification of target compound 4-11
[0078] use 1 H-NMR, 13 The chemical structures of the synthesized target compounds 4-11 were identified and confirmed by means of C-NMR, ESI-MS, and IR. The physical and chemical properties and spectral data of the eight compounds are shown in Table 1.
[0079] Table 1 Physicochemical constants and spectral data of compounds 4-11
[0080]
[0081]
Embodiment 3
[0083] Molecular docking of target compounds 4-11 with HDAC 2 and HDAC 8
[0084] The present invention uses the GLIDE program to dock the molecules of compound 4-11 with the crystal structure of HDAC 2 (PDB ID: 4LXZ) and the crystal structure of HDAC 8 (PDB ID: 3SFF); The docking effect, the Glide score results show that the docking scores of compound 4-11 and HDAC 2 are -7.627, -8.474, -8.079, -7.035, -7.999, -7.994, -7.655 and -7.788, and the docking scores with HDAC 8 They were -8.218, -9.022, -8.278, -8.269, -8.022, -8.177, -8.524 and -9.635; all 8 compounds could well bind to the active pockets of HDAC 2 and HDAC 8, and the lipids of 8 compounds The chain part occupies the long and narrow channel in the pocket, and the hydroxamic acid group is located at the bottom of the channel, and the hydroxamic acid group can be combined with the Zn at the bottom of the pocket. 2+ Form stable chelation; 8 compounds can form stable hydrogen bond interactions with amino acid residues...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com