Biological preparation method of mannose
A technology of mannose and glucose, applied in the field of mannose biological preparation, can solve the problems of lack of reference significance in authenticity, conversion rate value not given, low activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
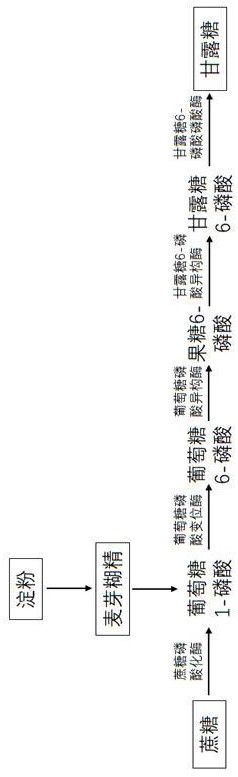
[0025] Example 1 Acquisition of M6PP gene and vector construction
[0026] Through database mining and functional characterization, 10 phosphatases from the genus Thermotoga were screened and obtained, which are mannose 6-phosphate phosphatases. The amino acid sequences of the phosphatases in the NCBI database are WP_101513072.1 (SEQID NO.1), WP_101510852.1 (SEQ ID NO.2), WP_041843828.1 (SEQ ID NO.3), WP_012644897.1 (SEQ ID NO.4), WP_008192576.1 (SEQ ID NO.5), WP_165304640.1 (SEQ ID NO. .6), WP_038054438.1 (SEQ ID NO.7), WP_012895992.1 (SEQ ID NO.8), WP_012310432.1 (SEQ ID NO.9), ABQ46309.1 (SEQ ID NO.10).
[0027] After the codon-optimized amino acid sequences of the above-mentioned mannose 6-phosphate phosphatases are codon-optimized, the corresponding gene nucleotide sequences are respectively as SEQ ID NO.11, SEQ ID NO.12, SEQ ID NO.13, SEQ ID NO.14, Shown in SEQ ID NO.15, SEQ ID NO.16, SEQ ID NO.17, SEQ ID NO.18, SEQ ID NO.19, SEQ ID NO.20. The gene sequence was sent to...
Embodiment 2
[0028] Example 2 Expression and purification of genes
[0029] The recombinant plasmids respectively containing the above 14 mannose 6-phosphate phosphatase genes were transformed into Escherichia coli for exogenous expression and purification.
[0030] Taking the phosphatase whose database number is WP_101513072.1 as an example,
[0031] (1) Transform E. coli expression recombinant plasmid pET-M6PP072 into E. coli BL21(DE3) to obtain recombinant bacteria.
[0032] (2) Pick a single clone into 5mL LB liquid medium, culture at 37°C, 220r / min until OD 600 0.6-0.8. Transfer the bacterial liquid in 5mL LB medium to 800mL 2YT medium, and cultivate to OD at 37°C and 220rpm 600 When the temperature is 0.6-0.8, cool down to 16°C, add IPTG to a final concentration of 0.5mM, and induce expression for 16h.
[0033] (3) Collect the above cultured bacteria into a 50 mL centrifuge tube, and centrifuge at 5500r / min for 15min;
[0034] (4) Discard the supernatant, and resuspend the cells...
Embodiment 3
[0037] Example 3 Determination of M6PP enzyme activity
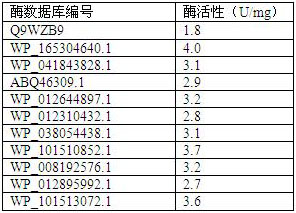
[0038] For the M6PP purified above, its dephosphorylation activity against mannose 6-phosphate was measured respectively. The enzyme activity assay system included the following: mannose 6-phosphate (20mM), Mg 2+ (1mM), phosphate buffer (pH 7.5, 50 mM), enzyme (0.5 mg / mL), 55°C, react for 10min. Enzyme activity definition (1U): The amount of mannose catalyzed per minute per mg of M6PP is 1 micromole. The samples were detected by high performance liquid chromatography.
[0039] The enzymatic activity of M6PP obtained by calculation and analysis is shown in Table 1. It was found that the 10 phosphatases screened were WP_165304640.1; WP_041843828.1; ABQ46309.1; WP_012644897.1; ; WP_008192576.1; WP_012895992.1 and WP_101513072.1, the catalytic activity of which is derived from Thermotoga maritima published in patent CN201910126884.8 Thermotoga maritima Compared with M6PP (TmM6PP) (Q9WZB9), all have different degrees of i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com