Gefitinib pharmaceutical co-crystal
A gefitinib and co-crystal technology, applied in the field of gefitinib drug co-crystal and its preparation, can solve the problems of low stability, limited application, research on co-crystal properties, etc., and achieve stable dissolution rate and solubility characteristics. Good, high dissolved concentration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
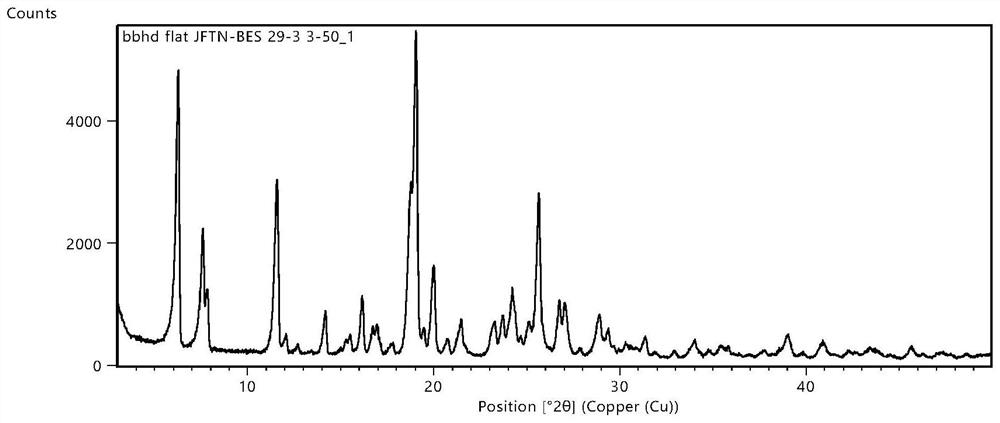
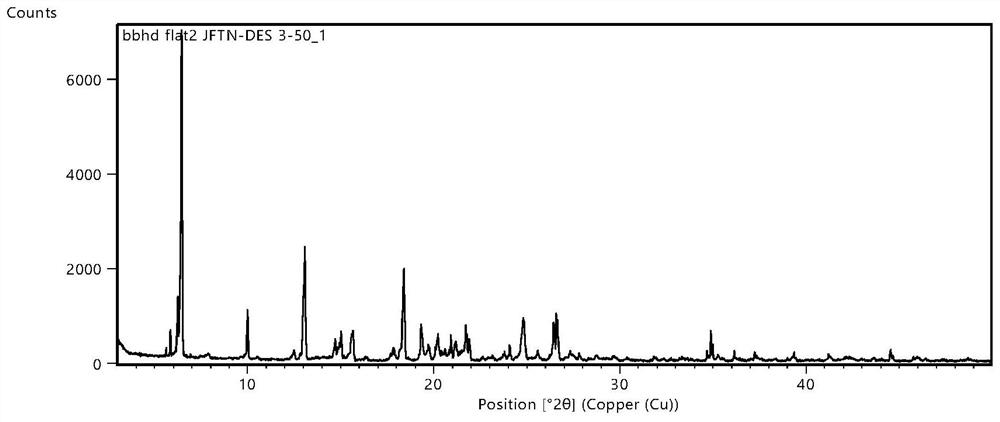
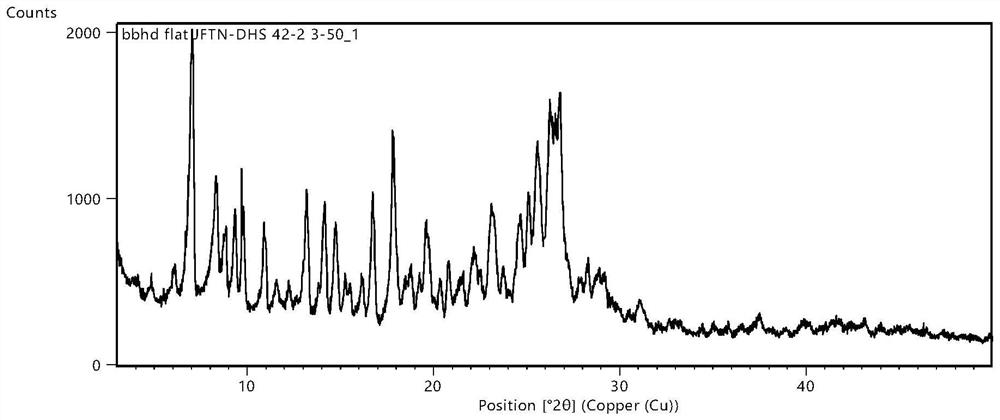
Image
Examples
Embodiment 1
[0132] 60.0mg of gefitinib and 27.9 mg of propylene acid were placed in a mortar, and 1 ml of methanol was added dropwise thereto, stirring with 35min, then adding 5 mL of methanol to continue to ground 15min, resulting in a clear solution, temperature control 0 ~ 5 ° C, static The crystal was added 48 hours, filtered, dried at 50.5 ° C for 8 h, resulting in the polycryrocarin composed of gefitinib and malonic acid in molar ratio 1: 2, yield 94.32%, HPLC purity 99.96%.
Embodiment 2
[0134] 50.0mg of gefittoribi and 23.9 mg of propylene acid were placed in a mortar, and 1 ml of ethanol was added dropwise thereto, stirring 30 min, and then 3 ml of ethanol continued to ground 10 min, resulting in a clear solution, temperature control 5 ~ 10 ° C The crystallization was allowed to concentrate 52 hours, filtered, dried at 55 ° C for 10 h, resulting in the polycrystin made of gefitinib and malonic acid in molar ratio 1: 2, yield 95.76%, HPLC purity 99.97%.
Embodiment 3
[0136] 80.0 mg of gefitinib and 41.0 mg of propylene acid were placed in a mortar, and 1 ml of methanol was added dropwise thereto, stirring with 10 ml of ethanol to continue grinding 20 min, resulting in a clear solution, temperature control 0 ~ 5 ° C , The crystal was allowed to concentrate 72 hours, filtered, dried at 60 ° C for 9 h, resulting in the eutectic acid composed of gefitinib and propanedic acid in molar ratio 1: 2, yield 94.14%, HPLC purity 99.95%.
[0137] Second, the preparation method of the common crystals composed of molar ratio 4: 1 in molar ratio 4: 1:
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap