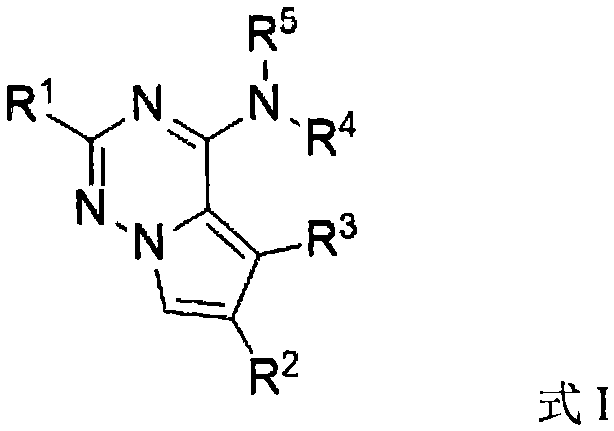
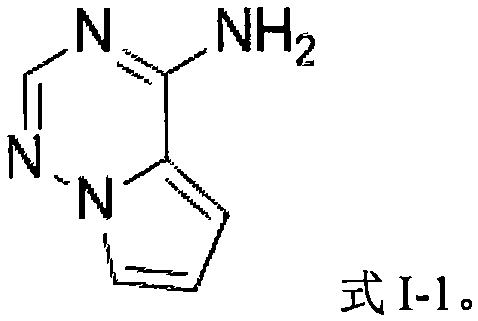
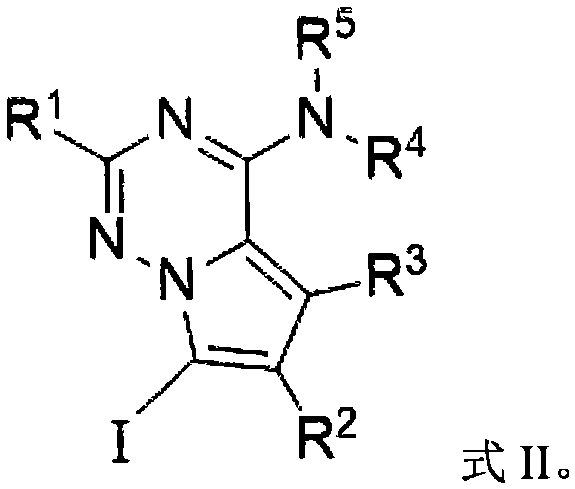
Preparation method and application of iodo-pyrrolotriazine amine compound
A technology for amine compounds and iodopyrrole, which is applied to the preparation method and application field of iodopyrrolotriazine amine compounds, can solve the problems of separation and purification of target products, and the reaction is not green enough, and achieves mild reaction conditions, The effect of simple post-processing and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Preparation of 7-iodopyrrolo[2,1-F][1,2,4]triazin-4-amine
[0066] Add sodium iodide 0.5mmol (1.0equiv), diphenyl sulfoxide 1.5mmol (1.5equiv) and toluene (as solvent) in the reaction bottle, add hydrochloric acid (2.5equiv.) under stirring, then place the reaction bottle in the Stir in an oil bath at 30°C for 1.0h. Then add pyrrolo[2,1-F][1,2,4]triazin-4-amine 0.5mmol (1.0equiv, make the substrate concentration 0.05M) to it, then heat to 65°C, and detect the reaction until After the raw material is consumed, it is quenched after cooling to room temperature, extracted with ethyl acetate, dried, and the solvent is removed by distillation under reduced pressure to obtain the crude product 7-iodopyrrolo[2,1-F][1,2,4]triazine -4-amine, the calculated yield of the target product is 52%.
[0067] 1 H NMR (400MHz, DMSO-d 6 )δ7.91(s, 1H), 7.79(br s, 2H), 6.97(d, J=4.4Hz, 1H), 6.80(d, J=4.4Hz, 1H). 13 C NMR (101MHz, DMSO-d 6 )δ155.7, 149.2, 118.8, 118.2, 104.4, 71.9. MS m / ...
Embodiment 2
[0069] Preparation of 7-iodopyrrolo[2,1-F][1,2,4]triazin-4-amine
[0070] Same as Example 1, only change the reaction solvent, the reaction solvent is acetonitrile (making the substrate concentration 0.05M) the yield of the target product, 63%.
Embodiment 3
[0072] Preparation of 7-iodopyrrolo[2,1-F][1,2,4]triazin-4-amine
[0073] Same as Example 1, only change the reaction solvent, the reaction solvent is tetrahydrofuran (to make the substrate concentration 0.05M), the yield of the target product: 47%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap