Method, device and equipment for generating clinical test scheme based on BOIN design decision
A technology for clinical trials and design decisions, applied in the field of clinical trials, can solve the problems of increasing human costs and long time consumption of clinical trials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0038] In order to make the purpose, technical solution and advantages of the present invention clearer, the technical solution of the present invention will be described in detail below. Apparently, the described embodiments are only some of the embodiments of the present invention, but not all of them. Based on the embodiments of the present invention, all other implementations obtained by persons of ordinary skill in the art without making creative efforts fall within the protection scope of the present invention.
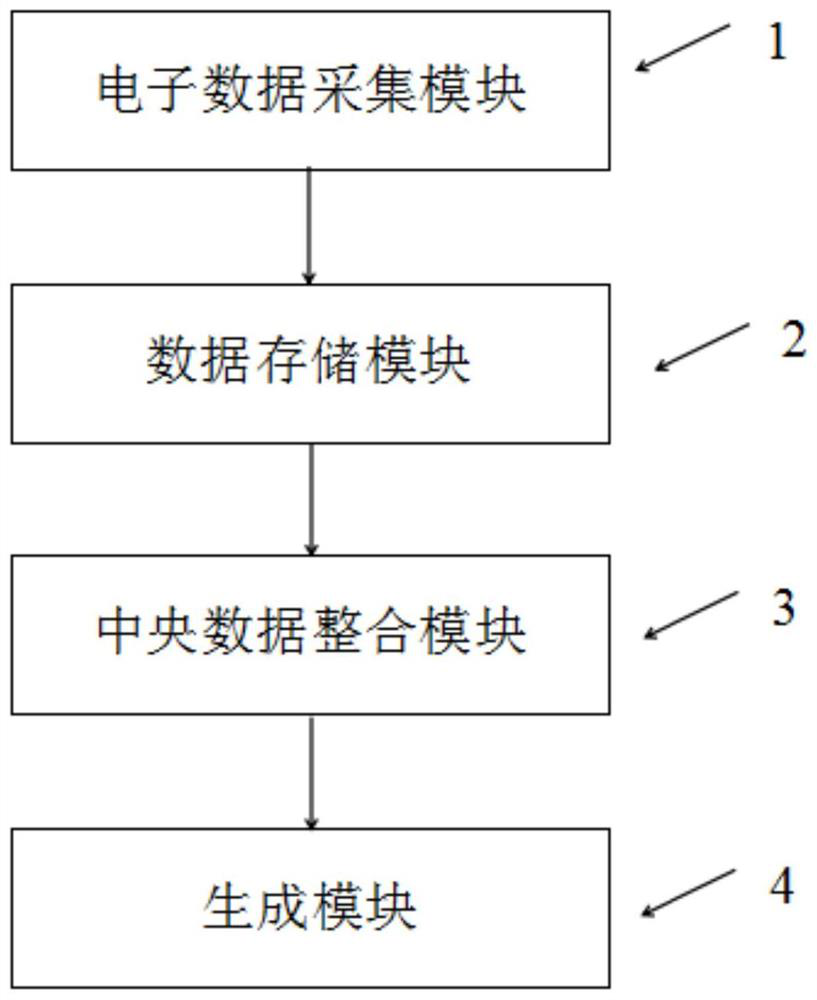
[0039] A kind of method based on Bayesian optimal interval (BOIN) design decision-making clinical trial scheme, is characterized in that, comprises:
[0040] S11. Acquiring basic data for generating a clinical trial plan based on BOIN design decision;
[0041] The Bayesian optimal interval design is referred to as BOIN design for short.
[0042] In practical applications, the basic data can be collected through an electronic data acquisition system, and the el...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com