Product produced from off-gas stream containing H2S, SO2 and/or NH3
A gas flow and gas chromatography technology, applied in the direction of thiosulfate/dithionite/polythionate, ammonium sulfide/polysulfide, calcium fertilizer, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1: From tail gas to crude metal sulphide solution and further derivatives
[0092] This example shows the use of H 2 A process for the production of ammonium sulfide from a gas stream of S and refinery hydrocarbon pollutants, which is then used to produce ammonium thiosulfate. Sulfur-based salt solutions produced from industrial waste gases and fluids (refinery sour gas) are typically produced via crude sulphide solutions. Sour water stripping gas (SWSG) and / or acid gas (AG) can be converted into a metal sulfide solution, which can then be further converted into the corresponding sulfate salt. Furthermore, thiosulfates can be prepared from such metal sulfide solutions.
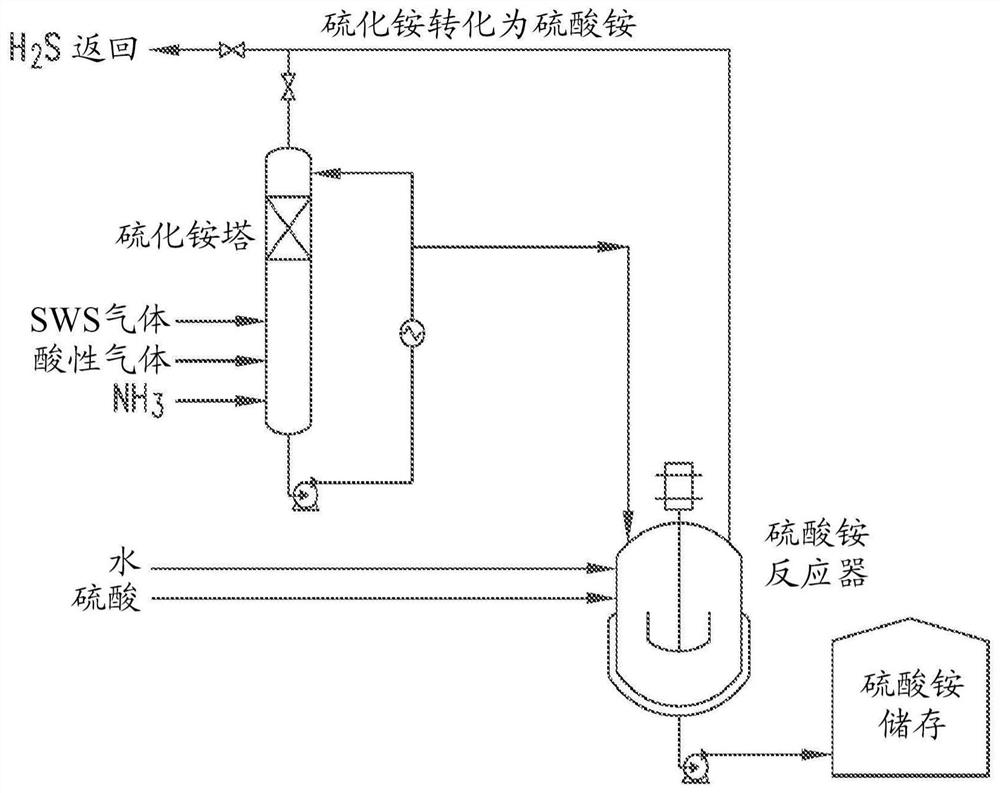
[0093] The reaction for preparing crude ammonium sulfide solution is shown below, but potassium sulfide solution can be prepared in a similar manner. figure 1 A schematic of a suitable method is shown. Sour water stripping gas usually contains about 1 / 3 ammonia (NH 3 ), about 1 / 3 of hydro...
Embodiment 2
[0103] Example 2: From tail gas to thiosulfate via (bi)sulfite intermediate
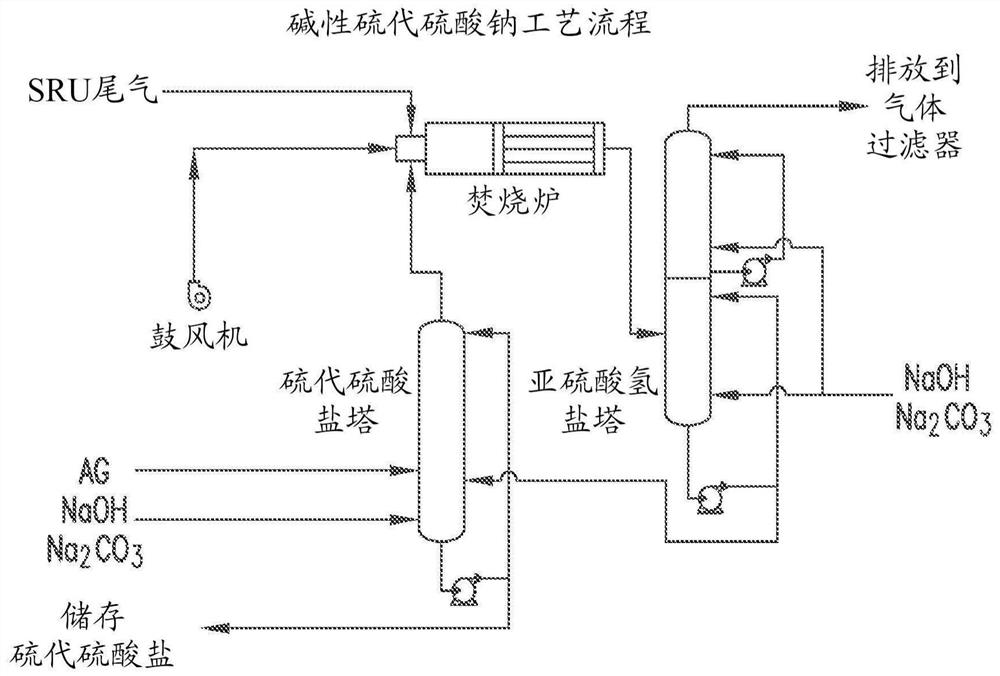
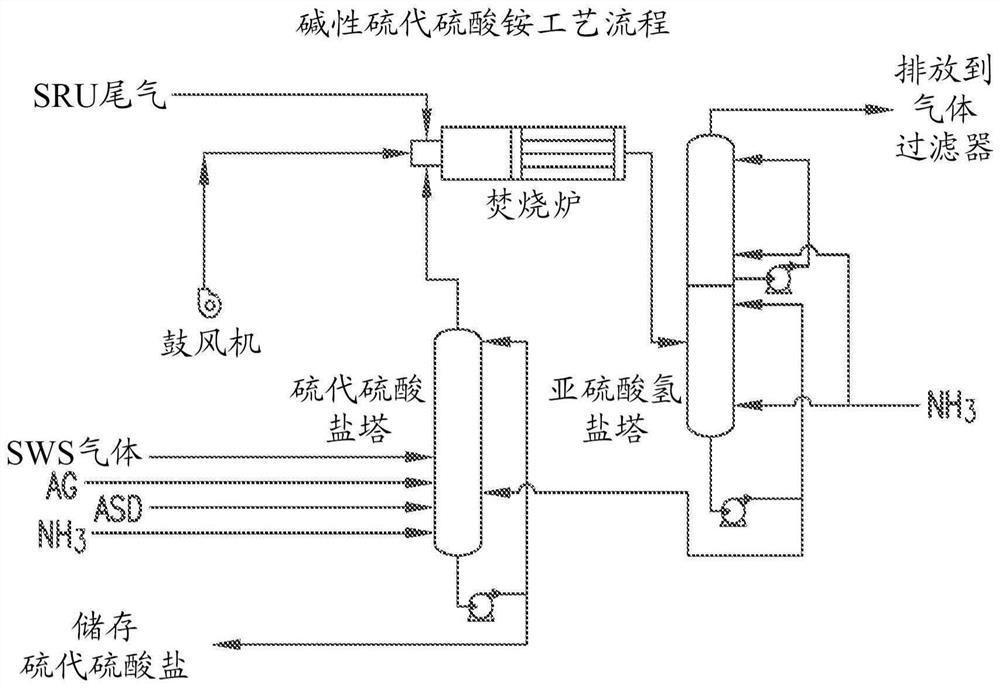
[0104] This example shows the use of H 2 A process for the production of ammonium thiosulfate from a gas stream of S and refinery hydrocarbon pollutants via bisulfite. Acid gas (AG) from the refinery as described above is sent to the Claus reactor where it is converted to elemental sulfur (S) and SO 2 . Reactions (4), (5) and (6) involve displacing SO 2 Conversion to ammonium sulfite / ammonium bisulfite solution. image 3 The method is shown schematically.
[0105] SO 2 +2NH 3 +H 2 O→(NH 4 ) 2 SO 3 (4)
[0106] (NH 4 ) 2 SO 3 +SO 2 +H 2 O→2(NH 4 )HSO 3 (5)
[0107] NH 3 +SO 2 +H 2 O→NH 4 HSO 3 (6)
[0108] In this method, for example a 60% by weight sulfite / bisulfite solution can be prepared. The sulfite / bisulfite solution is used as a 2 S) and ammonia (NH 3 ) reaction to prepare the intermediate of ammonium thiosulfate, as shown in the following reaction (7). When H ...
Embodiment 3
[0116] Example 3: From (bi)sulfite to calcium and magnesium thiosulfate solutions
[0117] Calcium and magnesium thiosulfate solutions are typically prepared from slurries that are further converted into thiosulfate solutions. For example, a solution of magnesium thiosulfate can be formed as follows:
[0118] MgO+2SO 2 +H 2 O→Mg(HSO 3 ) 2 (10)
[0119] Mg(HSO 3 ) 2 +2S+MgO→2MgS 2 o 3 +H 2 O (11)
[0120] Reaction (10) involves Mg(HSO 3 ) 2 Synthesis of intermediates. The intermediate is a slurry, which typically includes Mg(HSO 3 ) 2 / MgSO 3 and Mg(OH) 2 / MgO mixture. For convenience, the intermediate refers to Mg(HSO 3 ) 2 , since this is the predominant species that has been measured in solution. Reaction (11) involves the use of this intermediate to prepare a magnesium thiosulfate solution. As shown above, the stoichiometric reaction produces one mole of water for every two moles of magnesium thiosulfate produced.
[0121] To prepare the magnesium ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com