Multivalent FZD and WNT binding molecules and uses thereof
A technology for binding molecules and binding sites, applied in chemical instruments and methods, anti-receptor/cell surface antigen/cell surface determinant immunoglobulins, peptides, etc., and can solve problems such as inability to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
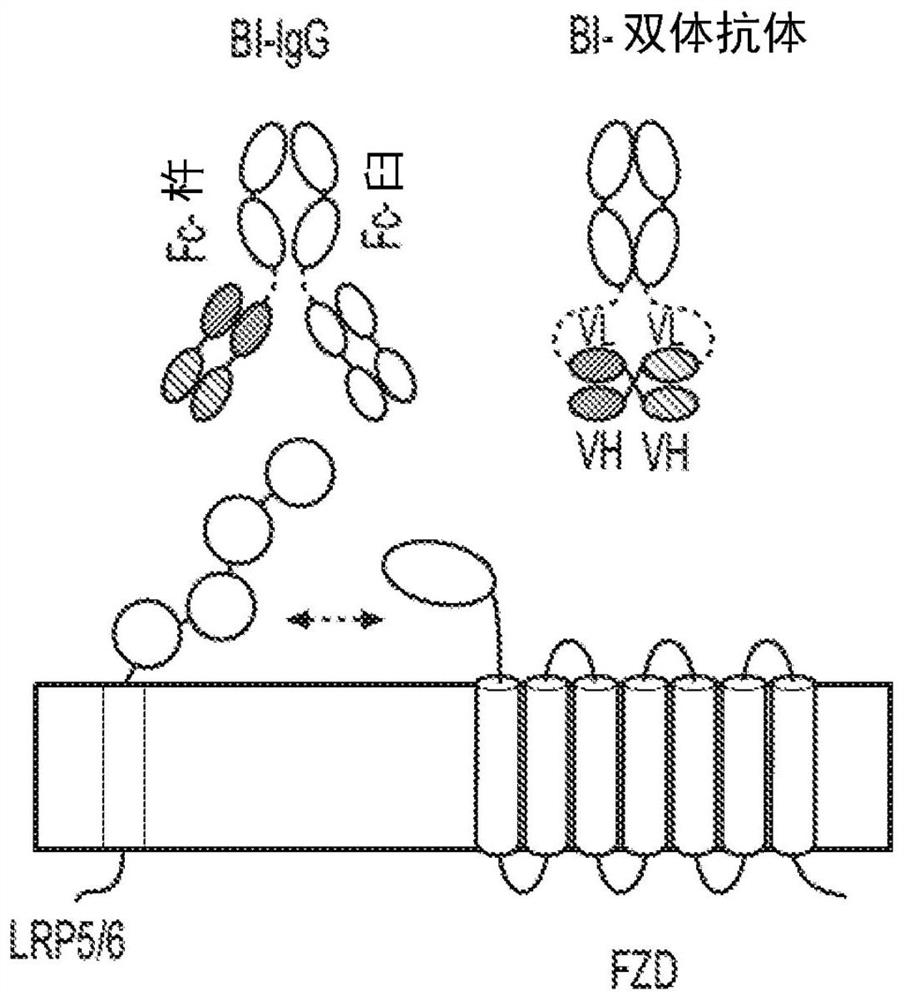
[0138] One embodiment of the present invention is a method for preparing a multivalent binding molecule that activates the Wnt signaling pathway, comprising:
[0139] a) selecting an Fc domain with a C-terminus and an N-terminus, such as that of an immunoglobulin comprising a CH3 domain, such as IgG, such as IgG1,
[0140] b) recognize antibodies with binding specificity for more than one FZD receptor and
[0141] c) recognizes an antibody with binding specificity to a Wnt co-receptor;
[0142] d) producing a nucleic acid molecule comprising
[0143] (i) a nucleotide sequence encoding the selected Fc domain,
[0144] (ii) a nucleotide sequence encoding the VL and / or VH of the antibody derived from step b, and
[0145] (iii) a nucleotide sequence encoding the VL and / or VH of the antibody derived from step c,
[0146] d) expressing the nucleic acid molecule of (d) to produce a polypeptide that forms a multivalent binding molecule through dimerization of the Fc domain, the mu...
Embodiment I
[0179] 1. Development of multivalent FZD agonists
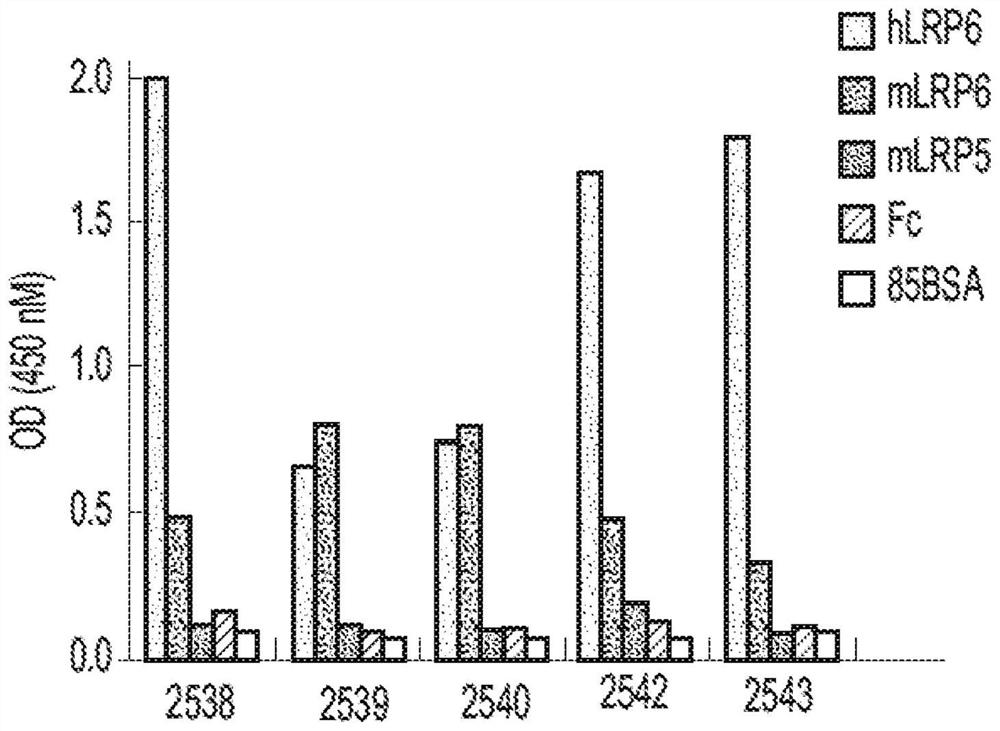
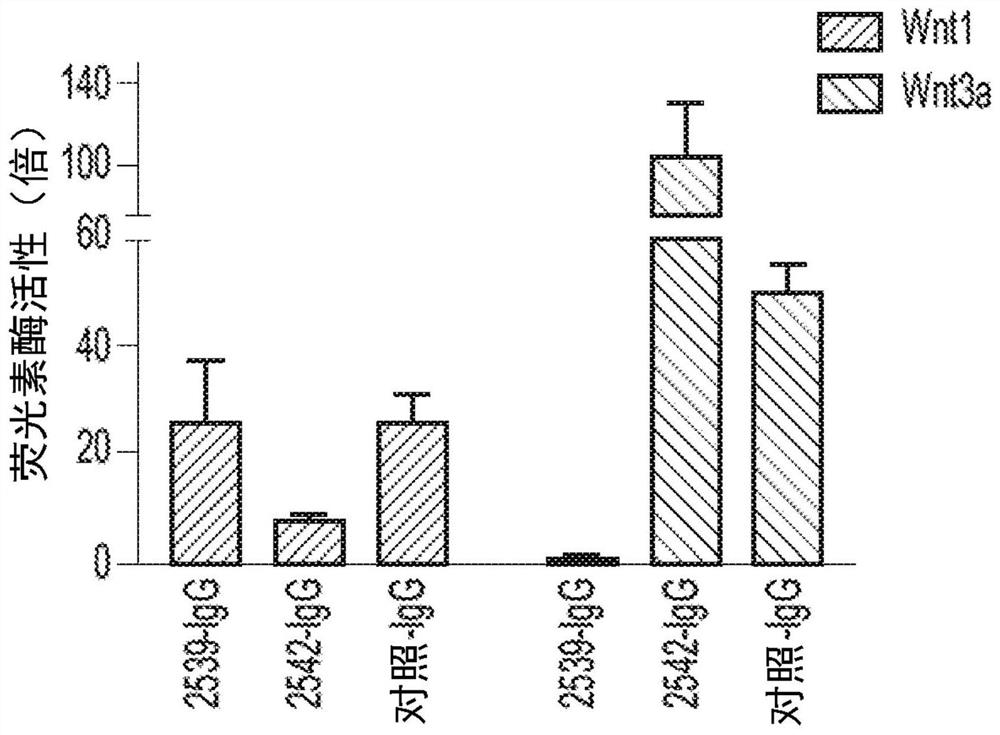
[0180] To prepare multivalent binding molecules with a first binding domain comprising a FZD diabody and a second binding domain comprising a co-receptor diabody, we selected the enriched half of the FZD receptor by using conventional phage display technology. Those bound by the cystine domain (CRD), FZD-specific antibodies were identified from a synthetic Fab phage library (Library F; see US Pub. No. 2016 / 0194394, inventor Sidhu et al.). Affinity or specific maturation is performed as desired. For example, pan-FZD binding antibody #5019 (recognizing FZD1, 2, 4, 5, 7 and 8) was matured from an FZD7-derived antibody using the FZD4 CRD as antigen. Our previous work also identified several antibodies fully specific to FZD4 (5038, 5044, 5048, 5062, 5063, 5080, 5081) or to FZD5 (2928) (see, for example, US20160194394, inventor Sidhu et al. and WO2017127933A1 , inventor Pan et al).
[0181] These FZD antibodies are used to prepa...
Embodiment II
[0220] Example II - Synthetic Antibodies Targeting FZD and LRP6
[0221] We previously applied phage display to generate hundreds of synthetic antibodies using nine recombinant FZD CRDs as antigens (the FZD3 CRD could not be purified) (Steinhart et al. Nat. Med. 23, 60 (2016); Pavlovic et al. MAbs (2018), doi: 10.1080 / 19420862.2018.1515565). System characterization revealed a continuous spectrum of specificity, with some Abs exhibiting broad specificity, such as the pan-FZD Ab (FP) recognizing FZD1 / 2 / 4 / 5 / 7 / 8 ( Figure 11A ), other Abs showed more restricted specificity, and some were monospecific ( Figure 11B ). Functional characterization revealed that some antibodies competed with Wnt and inhibited β-catenin signaling, while others were noncompetitive and did not interfere with Wnt signaling ( Figure 11B ). Altogether, we fully characterized 161 anti-FZD antibodies, including 47 Wnt signaling inhibitors. Unexpectedly, as discussed herein, regardless of whether they co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com