Endothelial lipase antibodies for treatment of cardiovascular diseases
A cardiovascular and antibody technology, applied in the direction of antibodies, antibody medical components, anti-enzyme immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0235] MEDI5884 was administered to cynomolgus monkeys in a single-dose non-Good Laboratory Practice (GLP) PK / pharmacodynamic (PD) study and 2 repeat-dose GLP toxicology studies. Single doses of MEDI5884 administered subcutaneously (SC) up to 30 mg / kg were well tolerated. There were no unplanned deaths, adverse clinical observations, injection site reactions, or adverse effects on body weight. Following repeated SC dosing (dose every 2 weeks) with 10, 30 or 100 mg / kg / dose of MEDI5884 for 1 or 6 months, all animals survived until scheduled sacrifice. No MEDI5884-related changes were observed in clinical observations, ophthalmic evaluations, body weight, behavior, neurophysiology, respiratory rate, injection site irritation score, heart rate, electrocardiogram (ECG), or blood pressure. Treatment-related findings were limited to pharmacologically mediated minimal to moderate increases in TC, HDL-C, LDL-C, and phospholipids at all dose levels. Microscopically, the presence of mi...
Embodiment 3
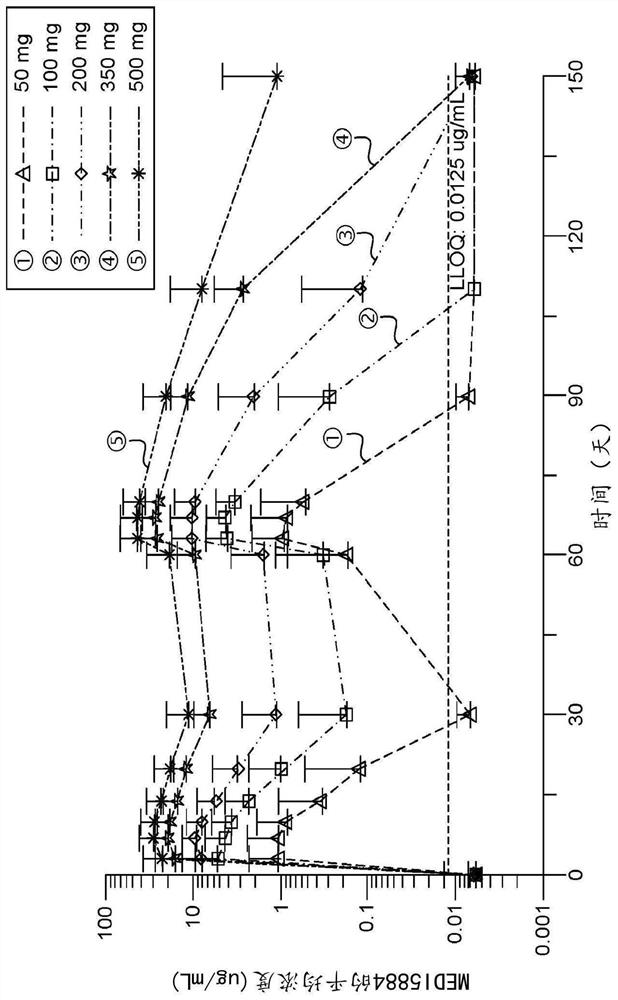
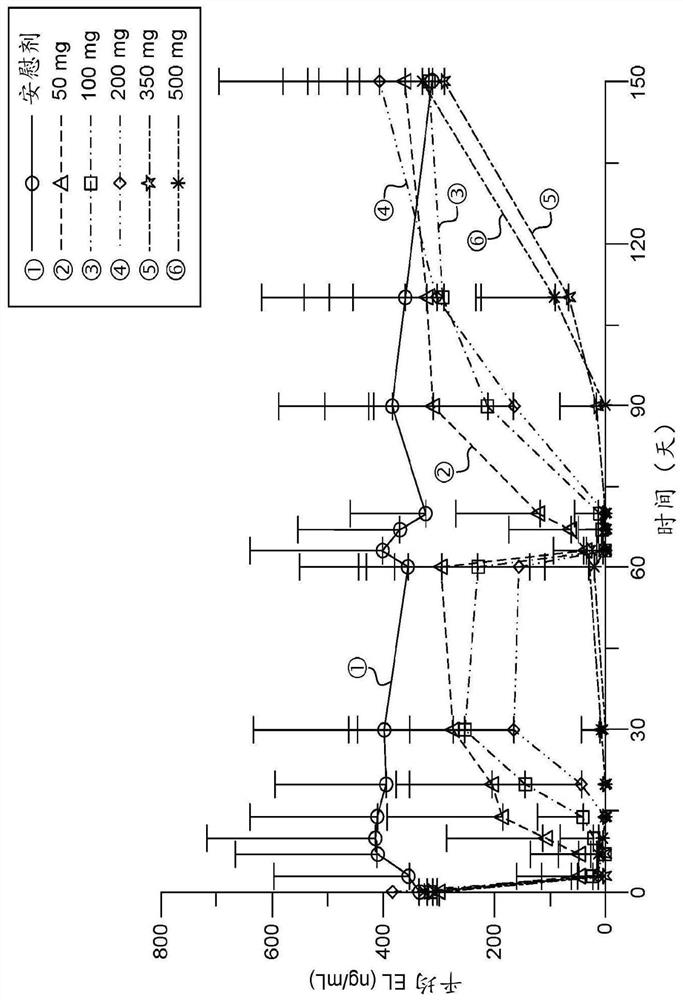
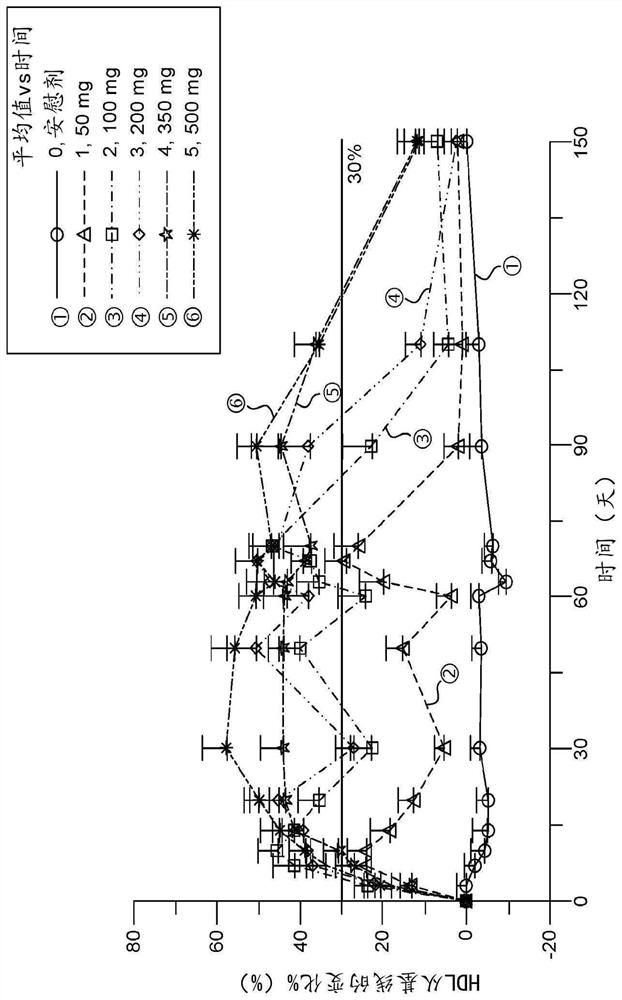
[0237] MEDI5884 was evaluated in a Phase 1, first-in-human blinded, placebo-controlled, single-dose escalation (SDE) study to evaluate the safety of subcutaneous (SC) administration of MEDI5884 in healthy subjects not receiving statin therapy Sex, PK and PD. Subjects were randomized in a 3:1 ratio to receive 30, 100, 300, or 600 mg of MEDI5884 or placebo; the initial cohort of each dose level was 6 MEDI5884 and 2 placebo subjects. These cohorts were subsequently replicated with subjects of Japanese ancestry to provide data to support clinical studies in Japan. A total of 64 subjects were enrolled at US sites. The duration of follow-up for each cohort varied from 28 days to 90 days after dosing.
[0238] Subjects received a single injection of MEDI5884 (or placebo) at doses of 30 and 100 mg, 3 SC injections of 300 mg dose (100 mg each) or 6 SC injections of 600 mg dose (100 mg each).
[0239] subjects
[0240] The median (SD) age of enrolled subjects was 35.9(9) years; 93...
Embodiment 6
[0315] A combined pharmacology study was performed to evaluate the effect of inhibition of proprotein convertase subtilisin / kexin type 9 (PCSK9) on lipoprotein metabolism following MEDI5884 treatment. The research program is in Figure 9 described in. To better simulate a hypothetical patient population already treated with LDL-C-lowering drugs, healthy cynomolgus monkeys were first given weekly subcutaneous injections of a PCSK9 neutralizing monoclonal antibody (mAb) (10 mg / kg; n=8) or Vehicle, starting on day 0, continued for four weeks to establish a baseline of low LDL-C. The PCSK9 mAb used was HS9 (comprising the VH and VL sequences of SEQ ID NO: 14 and 15, respectively). The HS9 antibody (in the context of the GLP-1 fusion protein known as MEDI4166) is disclosed in Chodorge et al., Sci. Rep. 8:17545 (2018) and International PCT Publication No. WO2015127273, each of which is incorporated by reference in its entirety and into this article.
[0316] Then on day 28 ( F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com