Phospholipid-FLAGLINE CONJUGATES AND METHODS OF USE THEREOF FOR TARGETED CANCER TREATMENT
A cancer, compound technology, applied in the field of phospholipid-flavagline conjugates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0174] chemical synthesis
[0175] PLE. PLE was synthesized according to Scheme 1 below:
[0176]
[0177] plan 1
[0178] Flavagline compounds. Flavagline is commercially available. FLV1 and FLV3 were purchased from Haoyuan Chemexpress Co. (Shanghai, China). FLV1 and FLV3 were alternately synthesized according to Scheme 2 below:
[0179]
[0180]
[0181] Scenario 2
[0182] PLE-flavagline construct. CLR 1852 (compound (9)) and CLR 1865 (compound (8)) were synthesized according to Scheme 3 below:
[0183]
[0184] Scenario 3
[0185] Synthesis of CLR 1899. CLR 1899 was synthesized according to Scheme 4. The structure was verified by NMR and MS data.
[0186]
[0187] Scenario 4
Embodiment 2
[0189] Presence of lipid rafts in tumor cells
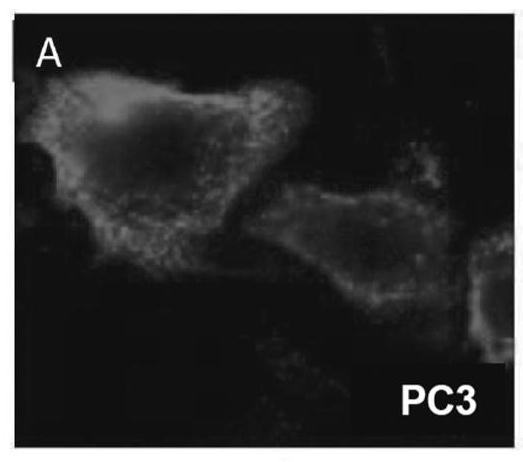
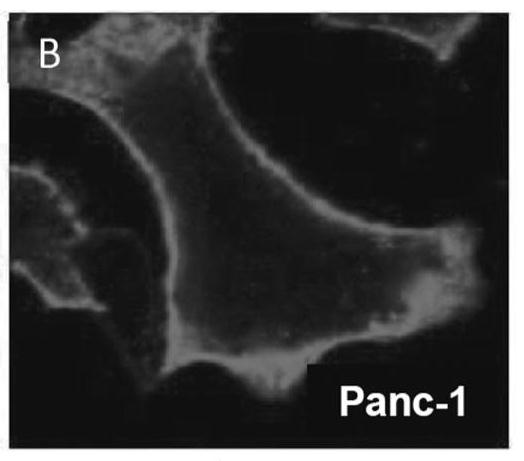
[0190] Over 100 cell lines were stained with cholera toxin subunit B, fixed with 4% formaldehyde, and stained with filipin III for 30 minutes. like Figures 1A-1D shown, nearly all tumor types tested showed high lipid raft concentrations in cell membranes (over 100 cell lines, fresh patient samples, etc.). like Figure 1E As indicated, A549 cells were co-cultured with normal fibroblasts for 48 h, then stained with cholera toxin subunit B, fixed with 4% formaldehyde, and stained with filipinIII for 30 min. These results suggest that tumor cells have higher concentrations of lipid rafts than normal cells.
Embodiment 3
[0192] PDC selects for uptake into tumor cells
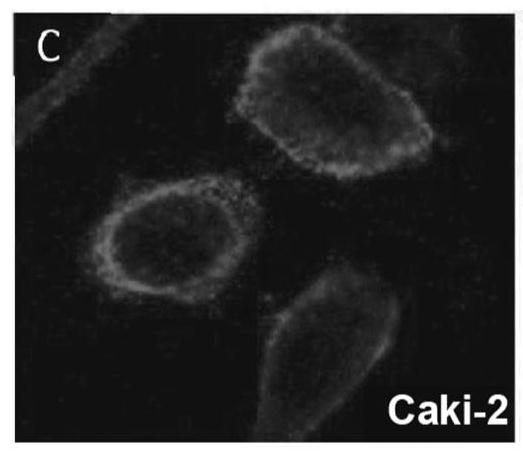
[0193] Normal fibroblasts and Caki-2 tumor cells (human clear cell renal cell carcinoma) were plated and co-cultured overnight ( Figure 1F ). Cells were then incubated with 5 μM CLR1501 (compound (1)) in complete medium at 37°C for 24 hours. Cells were washed the next day and co-stained with nuclear stain (Hoescht 33342). CLR 1501 was excited and then detected with an Alexa-Fluor 488 filter. CLR 1501 is highly localized in Caki-2 cells and least in normal fibroblasts.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com