Method for synthesizing boron nitride from aether boron trifluoride and lithium nitride
A technology of boron trifluoride diethyl ether and a synthesis method, which is applied in the fields of chemical instruments and methods, nitrogen compounds, inorganic chemistry, etc., can solve the problems of high price, high cost, and the toxicity of synthetic boron nitride raw materials, so as to reduce costs and increase reliability. operational effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] (1) Pour 50ml of benzene into an unlined stainless steel reaction kettle
[0014] (2) After grinding 5g of lithium nitride into a fine powder in a fume hood, put it into the reaction kettle
[0015] (3) Slowly pour 25ml of boron trifluoride diethyl ether into the reaction kettle in a fume hood, stir while pouring to make the raw materials evenly mixed, then seal the reaction kettle, and heat up to 300°C at a heating rate of 5°C per minute. 72 hours at temperature
[0016] (4) After the reactor is cooled, open the reactor to take out the reaction product. The reaction product was dissolved in deionized water and centrifuged to remove water-soluble substances, repeated three times, then soaked in 2mol / L dilute hydrochloric acid for 1 hour, washed with deionized water, and centrifuged three times. The final product is dried and ground to obtain the final reaction product.
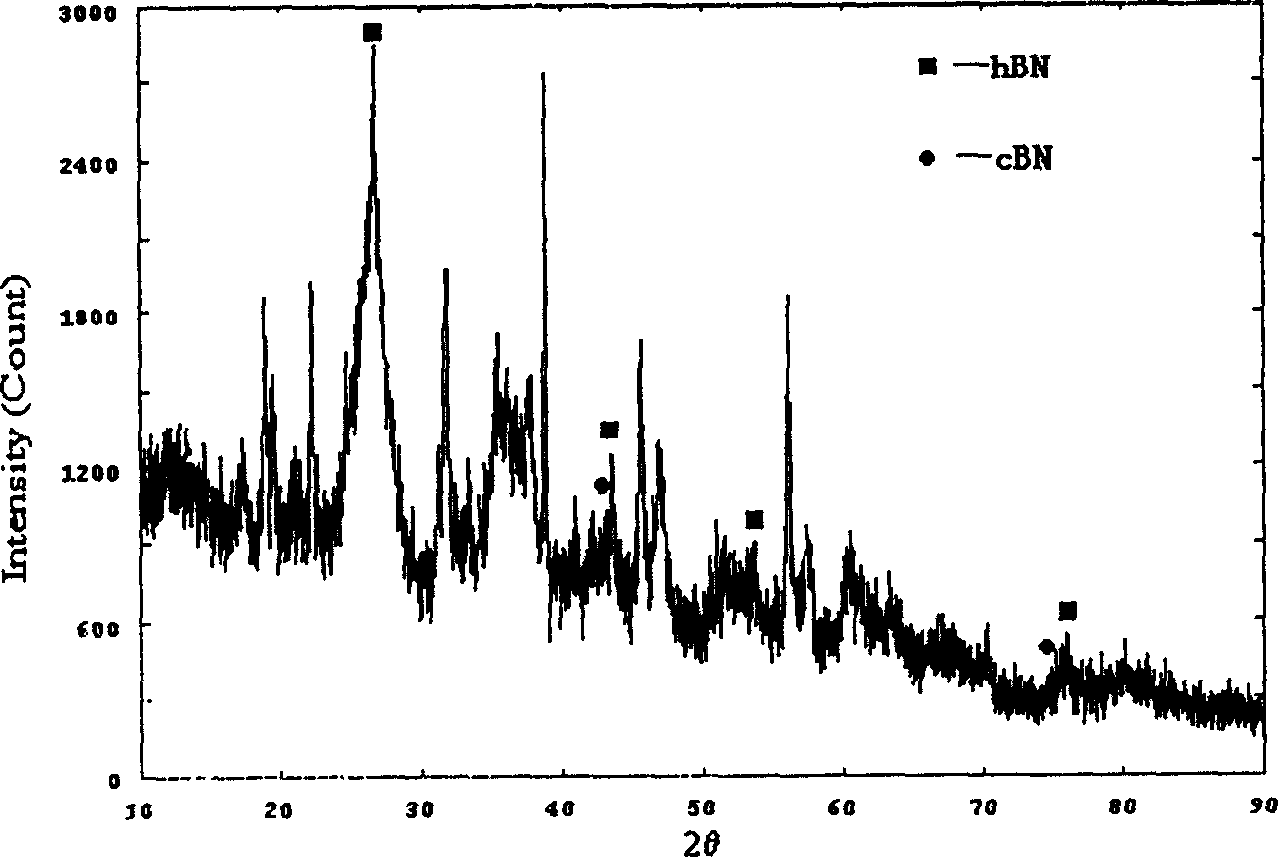
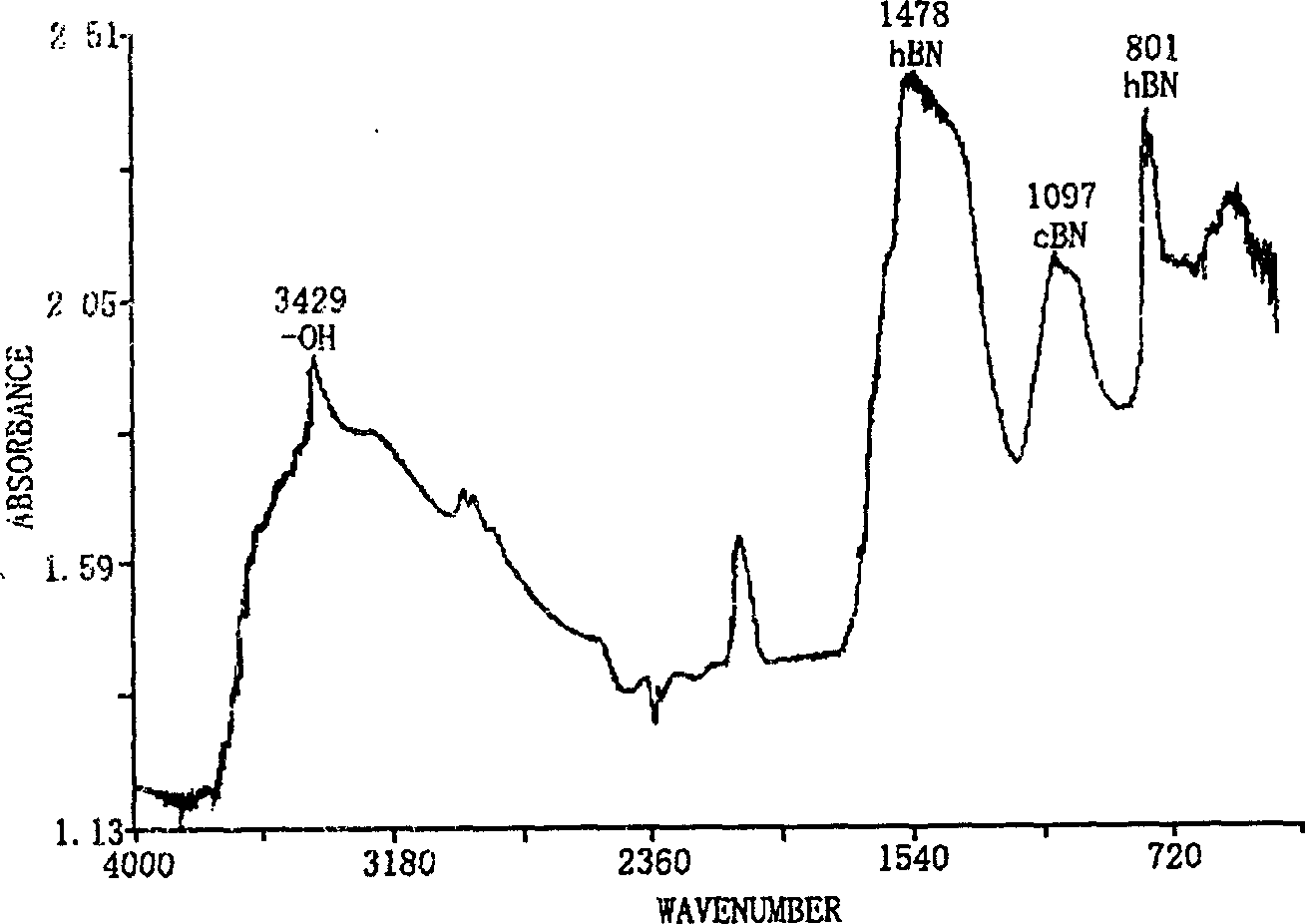
[0017] figure 1 and figure 2 Respectively are the X-ray diffraction spectrum and the Fourier t...
specific Embodiment 2
[0018] (1) Pour 50ml of benzene into an unlined stainless steel reaction kettle
[0019] (2) After grinding 5g of lithium nitride into a fine powder in a fume hood, put it into the reaction kettle
[0020] (3) Slowly pour 50ml of boron trifluoride diethyl ether into the reaction kettle in a fume hood, stir while pouring to make the raw materials evenly mixed, then seal the reaction kettle, and heat up to 400°C at a heating rate of 5°C per minute. 60 hours at temperature
[0021] (4) After the reactor is cooled, open the reactor to take out the reaction product. Dissolve the reaction product in deionized water and centrifuge to remove water-soluble substances, repeat the process 5 times, then soak in 2mol / L dilute hydrochloric acid for 1 hour, wash with deionized water, and centrifuge 3 times. The final product is dried and ground to obtain the final reaction product.
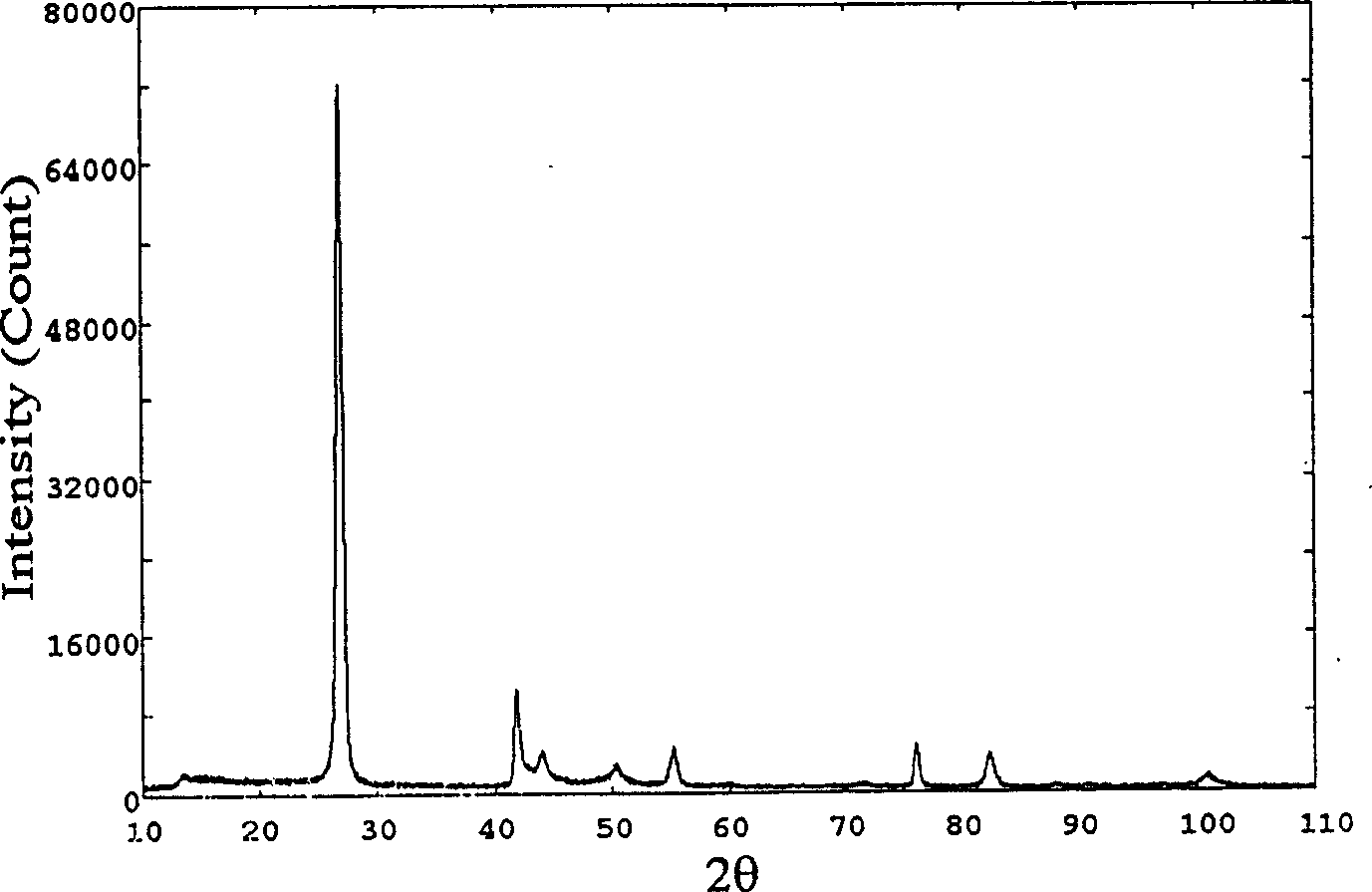
[0022] image 3 and Figure 4 They are the X-ray diffraction spectrum and Fourier transform infrared spe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com