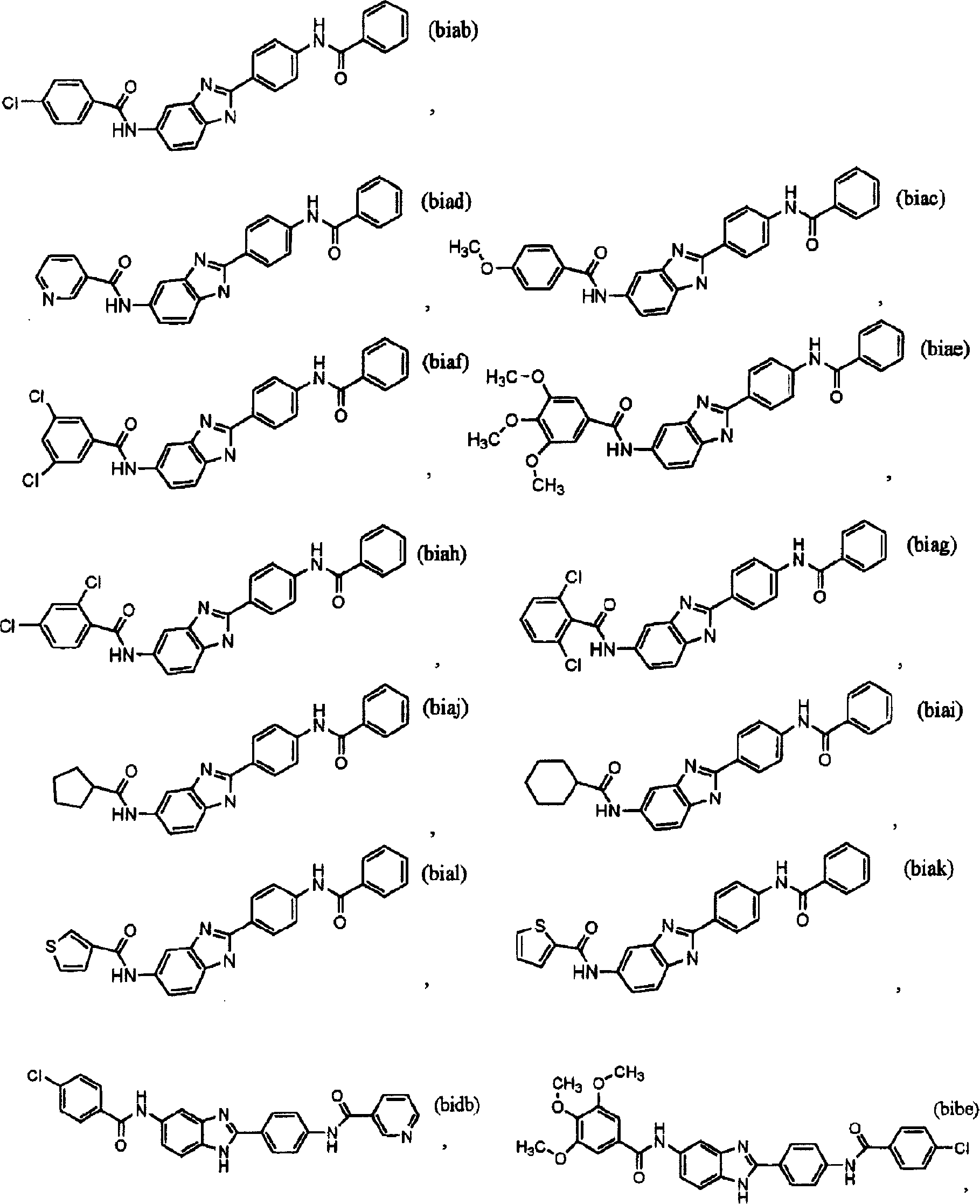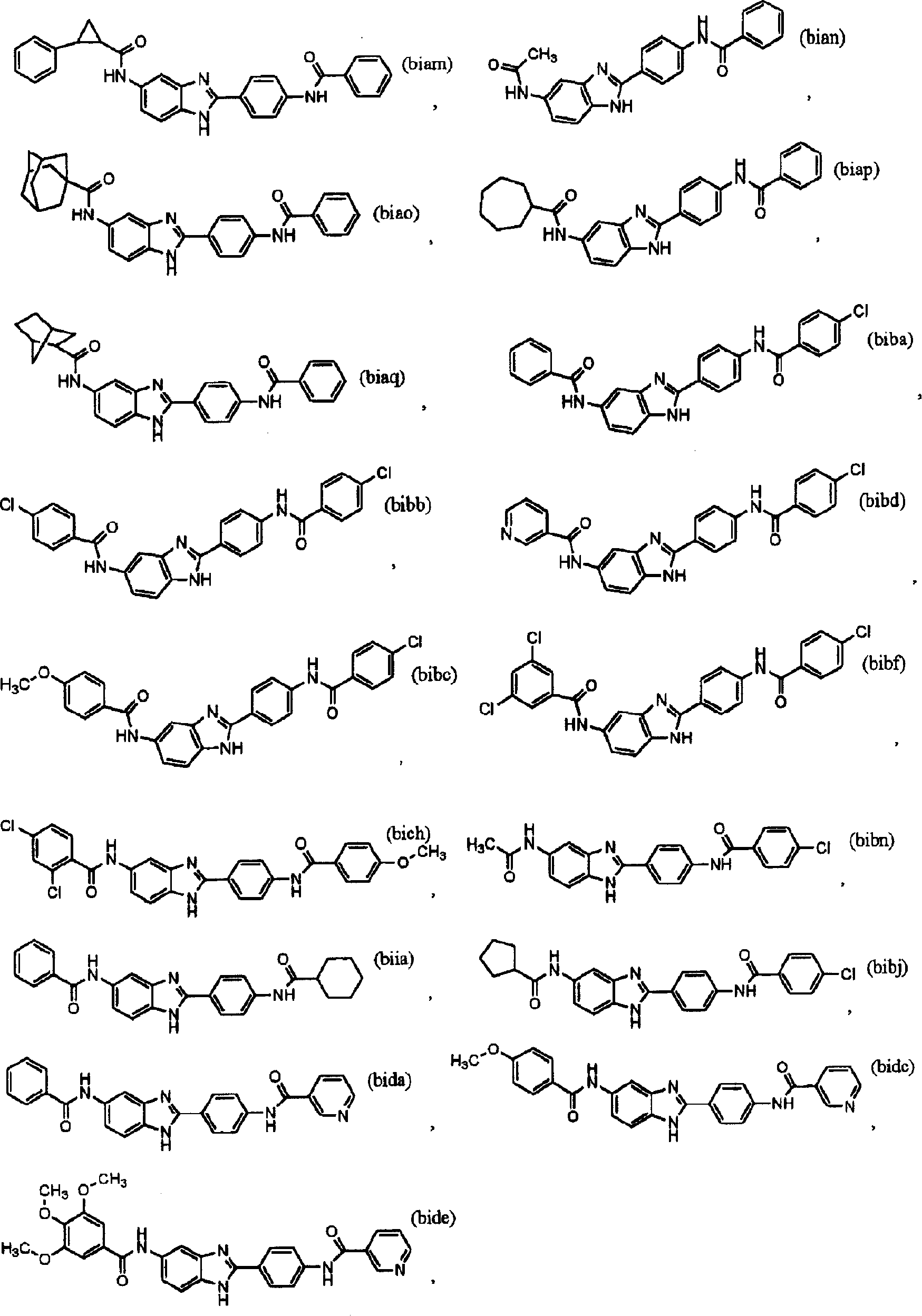Benzimidazole derivatives as modulators of IgE
A technology of immunoglobulin and drugs, which is applied in the direction of drug combinations, active ingredients of heterocyclic compounds, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
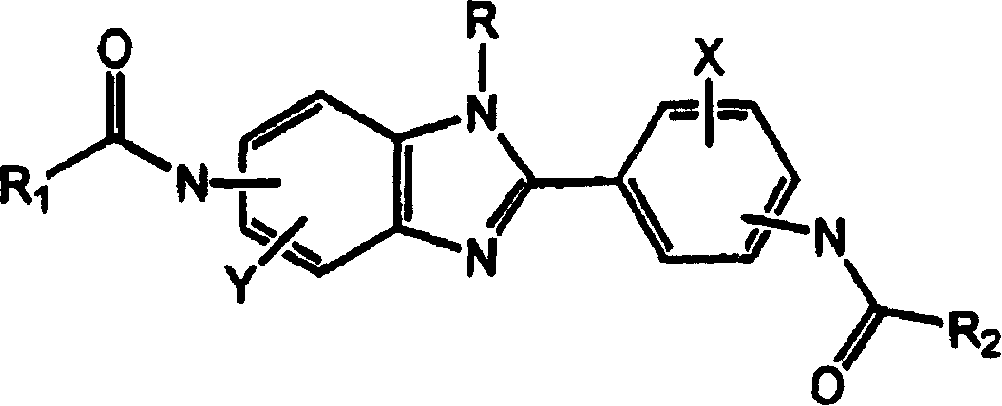
[0046] The present invention relates to small molecule IgE inhibitors (synthesis and / or release) useful in the treatment of allergy and / or asthma or any other disease in which IgE is the causative agent. Specific compounds disclosed herein are identified by their ability to inhibit IgE levels in in vitro and in vivo assays. By reference to the in vitro and in vivo assays described below, one skilled in the art can understand the evolution and optimization of clinical treatment regimens.
[0047] In vitro analysis
[0048] The assay begins with in vivo antigen priming and the secondary antibody response is measured in vitro. The basic protocol has been documented and has been optimized for a range of parameters including: antigen dose and length of priming, number of cells cultured in vitro, eliciting secondary IgE (and other Ig) responses in vitro antigen concentration, fetal bovine serum (FBS) batches to optimize IgE in vitro responses, the importance of priming CD4+ T cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com