Method for refinishing trimethylamine
A purification method and technology of trimethylamine are applied in the field of purification of high-purity trimethylamine, and can solve the problems of complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
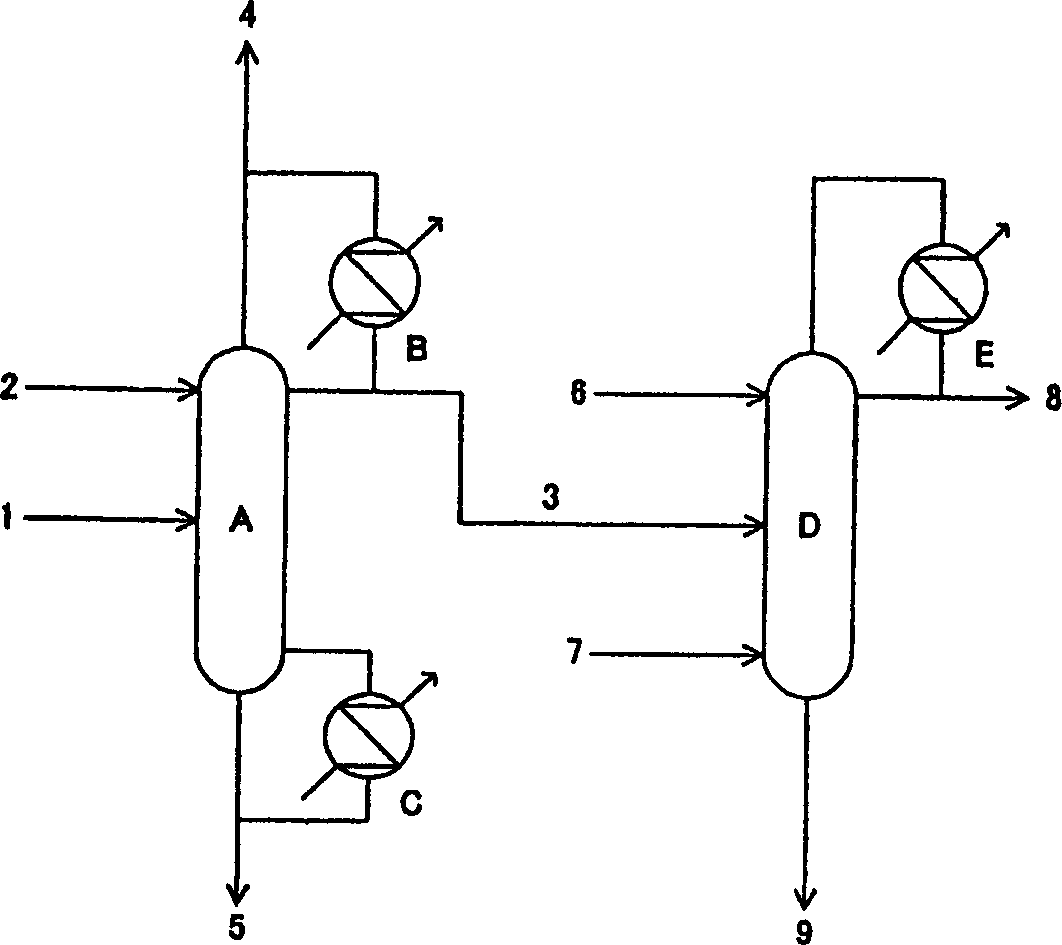
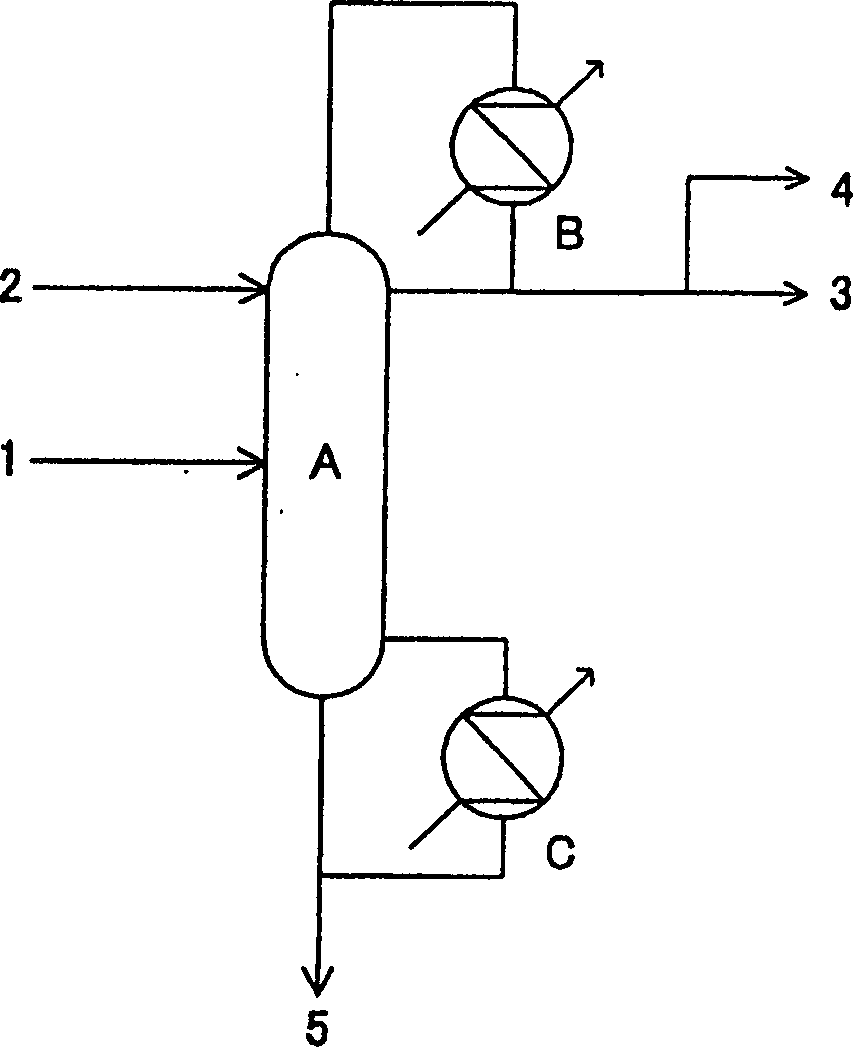
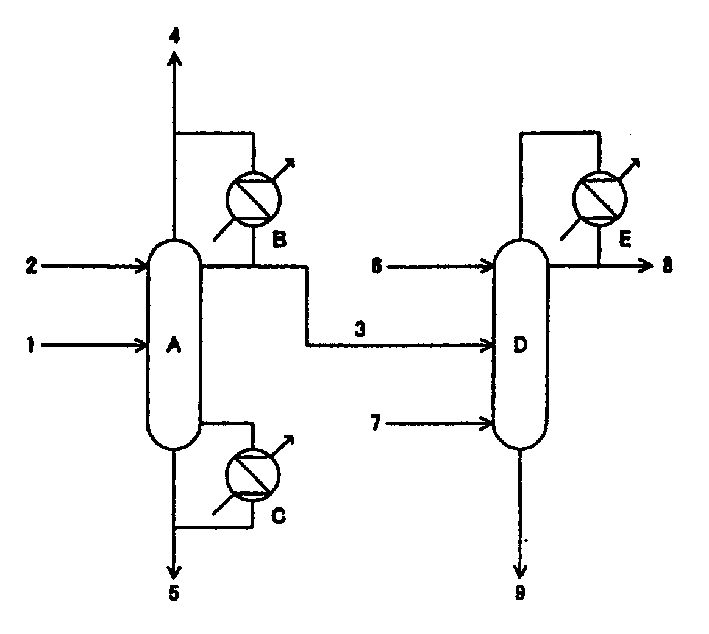
[0030] Methanol and ammonia carry out gas phase contact reaction in the presence of zeolite catalyst, and the reaction product obtained after removing ammonia in the ammonia separation tower is obtained at a rate of 1000 kg / hour, containing 11.0% by mass of monomethylamine, 40.9% of dimethylamine, and trimethylamine. 8.5%, methanol 5.3% and water 34.3% aqueous solution. This aqueous solution is used as figure 1 Distillation was performed as indicated. First, remove the middle section of the aqueous solution of ammonia supply trimethylamine separation tower (0.35 meters in tower diameter, the packed column that number of theoretical plates is 30), supply water with 722.3 kilograms / hour rate above this supply section, and the pressure at the top of the tower is Distillation was carried out under the conditions of 0.7Mpa and a reflux ratio of 9.8 (the reflux ratio=reflux flow / (column top gas extraction+extract liquid volume)). The overhead gas at this time was circulated in the...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap