Triazine-based compound comprising functionalized alkylthio groups, and photo polymerization initiator
A technology of alkylthio groups and triazine compounds, applied in the field of photosensitive compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Embodiment 1
[0046] The present invention is described in further detail using the following specific examples. However, these Examples are only for illustrating the present invention, and the present invention is not limited by these Examples. [Examples] Example 1: Preparation of 3-{4-[2,4-bis(trichloromethyl)-s-triazin-6yl]phenylthio}propionic acid represented by the following chemical formula 1a
[0047] [Chemical formula 1a]
[0048] [The first step: Synthesis of 3-(4-cyanophenylthio)propionic acid]
[0049]20g of 4-fluorobenzonitrile (165 mmol), 20g of 3-mercaptopropionic acid (188 mmol) and 50g of potassium carbonate were put into 300ml of DMF (dimethylformamide), and the bath temperature was maintained at 100°C for 20 Hour.
[0050] After the reaction temperature was lowered to room temperature, the reaction solution was slowly poured into 1500 ml of distilled water, and then the resulting precipitate was removed by filtration. The filtrate was acidified with 2N hydrochloric a...
Embodiment 3
[0070] Example 3: Preparation of ethyl 2-{4-[2,4-bis(trichloromethyl)-s-triazin-6-yl]phenylthio}acetate represented by the following chemical formula 1c
[0071] [Chemical formula 1c]
[0072] [The first step: Synthesis of ethyl 2-(4-cyanophenylthio)acetate]
[0073] 15 g of 4-fluorobenzonitrile (124 mmol), 16 g of ethyl 2-mercaptoacetate (133 mmol) and 25 g of potassium carbonate were put into 100 ml of DMF (dimethylformamide), and the bath temperature was kept at 130° C. for 20 hours.
[0074] After the reaction temperature dropped to room temperature, the reaction solution was slowly poured into 700 ml of distilled water, extracted with ether, washed with distilled water, dried over anhydrous magnesium sulfate, and filtered. Next, the volatiles were evaporated to give the crude product. The crude product was purified by column chromatography using a mixed solvent of hexane and ethyl acetate (3:1 by volume) as eluent. The product structure was analyzed by NMR (nuclear m...
Embodiment 10
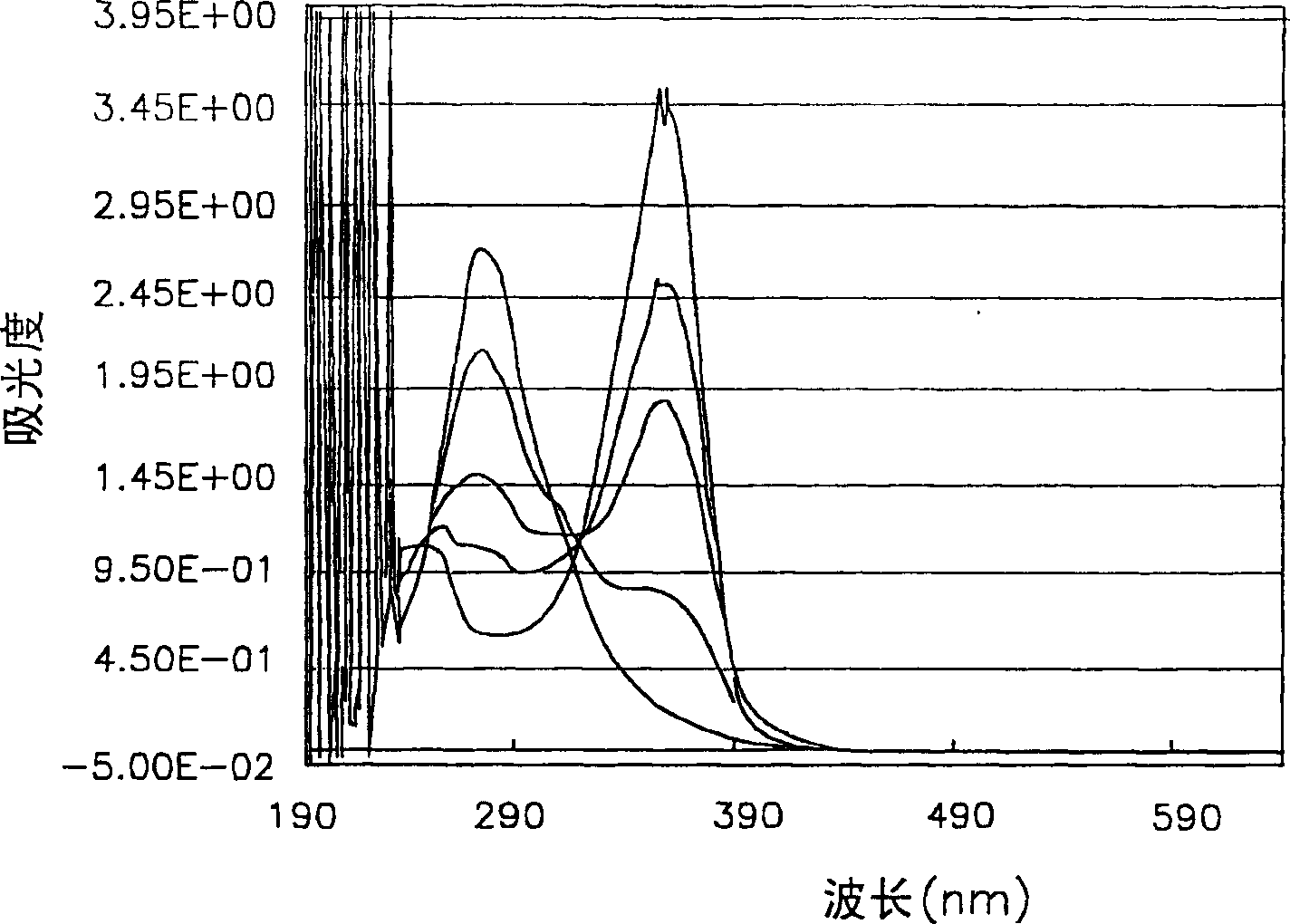
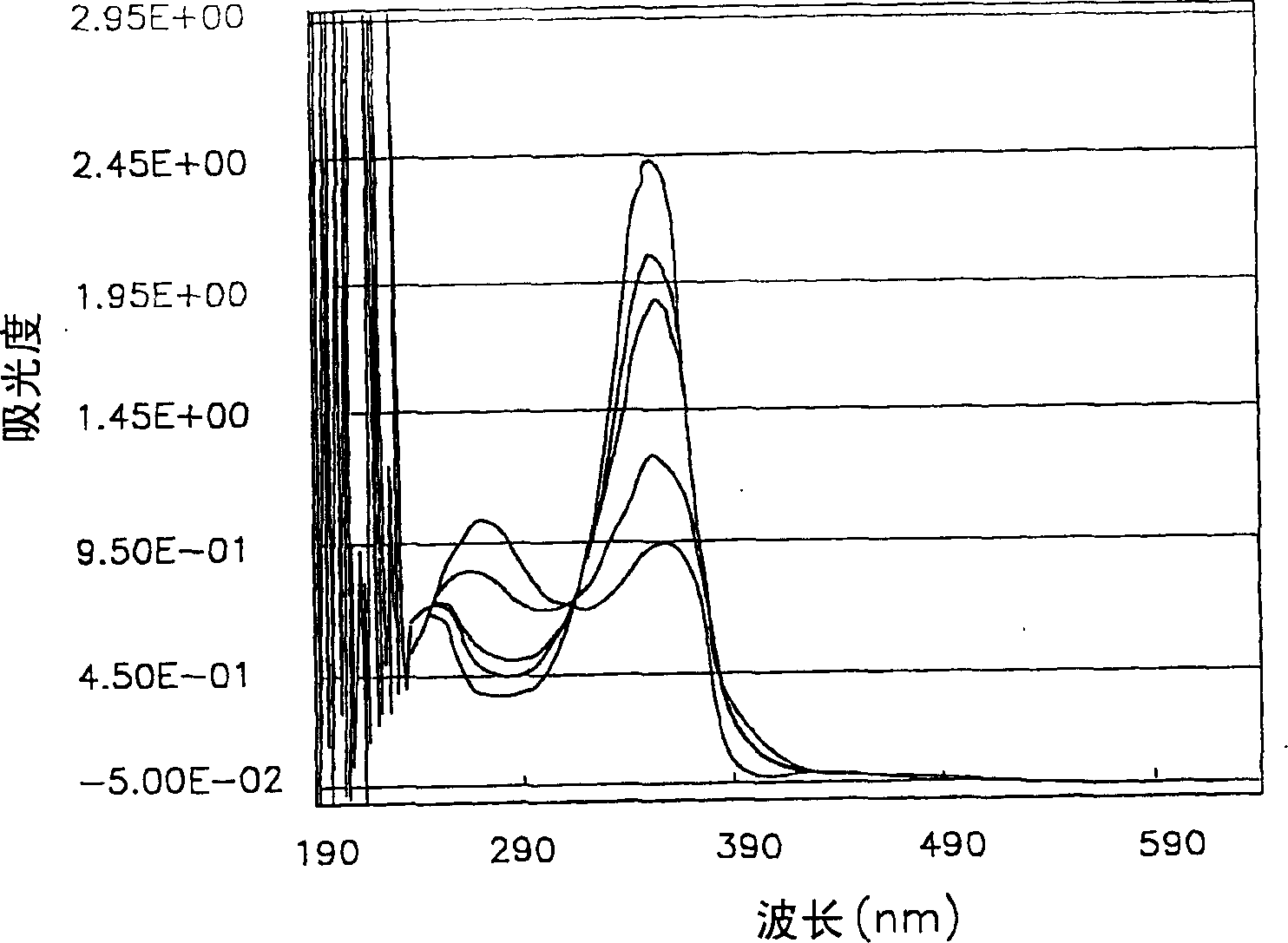
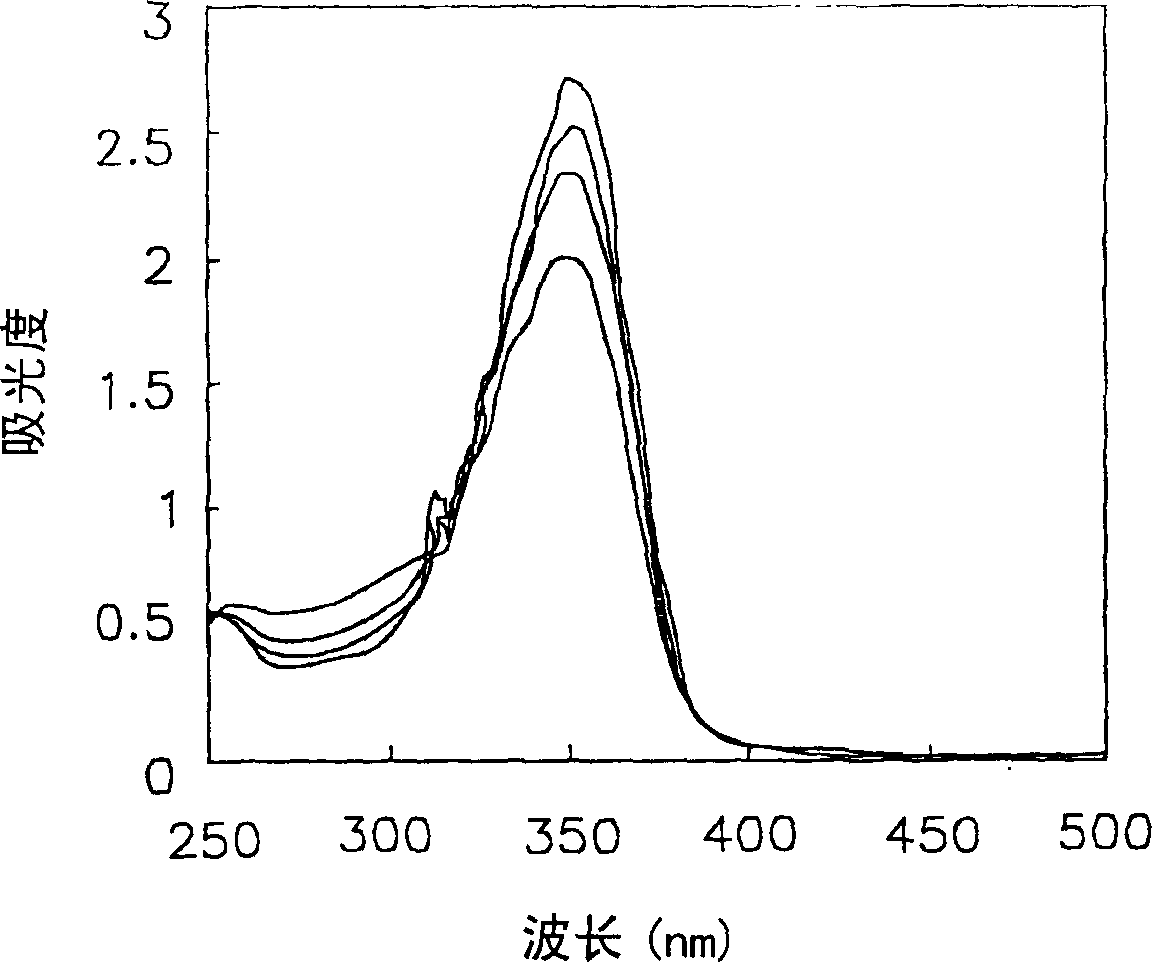
[0112] A photosensitive composition was prepared according to the same method as in Example 1, which contained the components shown in Table 10 below including the compound of Chemical Formula 1a of Example 1, and then the condition of the film on the glass surface was observed through an optical microscope, and it could be seen that a clear formation was formed graphics.
[0113] Classification
Raw material name
Content (parts by weight)
C.I. Pigment Red 177
90
BzMA:MAA=75:25
Mw: 35,000
50
polymerizable compound
Dipentaerythritol hexaacrylate
40
photopolymerization initiator
3-{4-[2,4-bis(trichloromethyl)-
s-Triazin-6-yl]phenylthio}propionic acid
30
Organic solvents
PGMEA (boiling point: 146°C)
790
[0114] A photosensitive composition was prepared according to the same method as in Example 1, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com