Nucleic acid and protein composition and its production method and application in immune regulation
A protein and composition technology, applied to nucleic acid substances and protein compositions and their production and application in immune regulation, can solve problems such as suppressing organ failure, achieve suppressing T cell immune responses, overcome nonspecific, Ease of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
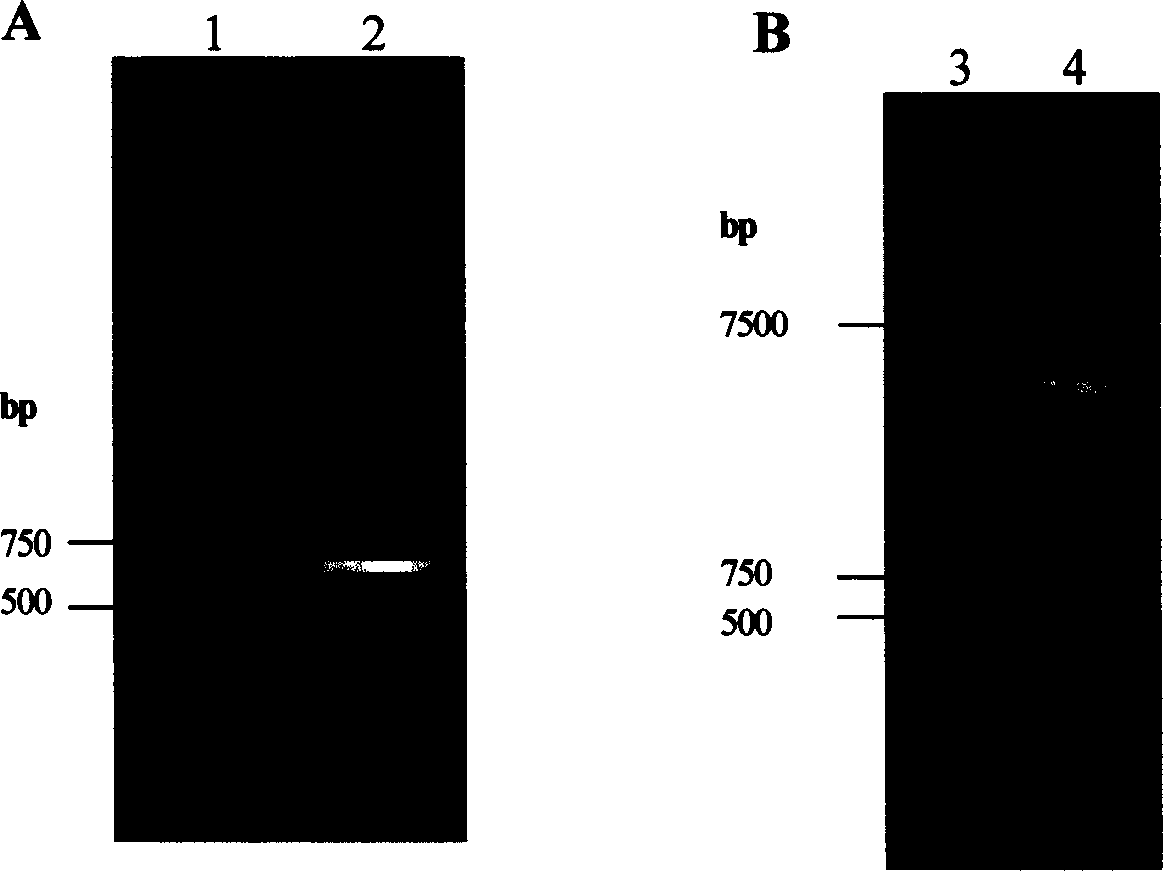
[0051] Preparation of Example 1 DNA
[0052] One method is to grind the animal tissue at low temperature, remove the protein in a phenol-chloroform solution, and separate the double-stranded DNA by ethanol precipitation.
[0053] Another method is to extract DNA from plant tissue by CTAB method, remove protein in phenol-chloroform solution, and separate double-stranded DNA by ethanol precipitation.
[0054] Another method is to extract plasmid DNA from Escherichia coli, remove protein in phenol-chloroform solution, and separate double-stranded DNA by ethanol precipitation.
[0055] The detailed description of the above extraction methods and techniques can be edited by "Molecular Cloning" (Second Edition 1998, Cold Spring Harbor Laboratory Press, New York) and Li Chaolong etc. in Sambrook et al., "Biochemistry and Molecular Biology Experimental Technology" Zhejiang University Publisher search.
Embodiment 2
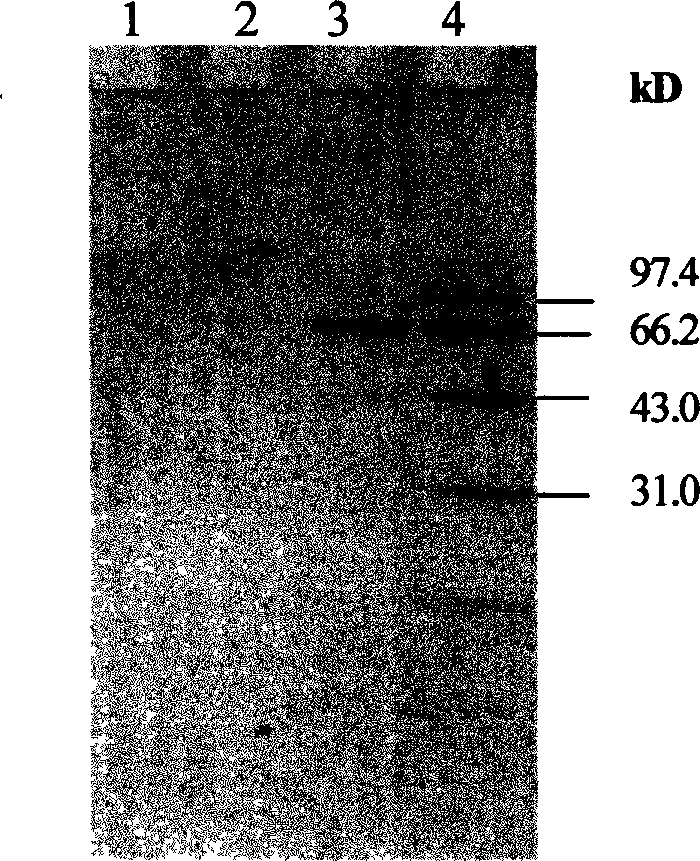
[0056] Embodiment 2: the preparation of protein and polypeptide
[0057] Proteins and peptides can be synthesized by standard automatic peptide synthesizers (such as ABI, 433A, etc.), according to the method used by the instrument manufacturer; or from animal tissues and cells, plant tissues and cells or microorganisms, according to conventional protein chemistry methods For extraction, it can also be extracted from genetically engineered expression bacteria or cells. These polypeptide extraction methods are well known and described in detail in "Protein Purification Protocols" by Doonan (1996, Humana Press, NJ).
Embodiment 3
[0058] Embodiment 3: preparation and inactivation of pathogen
[0059] Viruses, bacteria, mycoplasma, parasites, and allergens that are isolated and produced by pathogens are inactivated by known inactivation methods and reagents such as: formaldehyde or formalin, β-propiolactone, N-acetylethyleneimine, and dioxin Ethylene imine, etc., obtained by inactivating non-infectious pathogens, and then separated and purified.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com