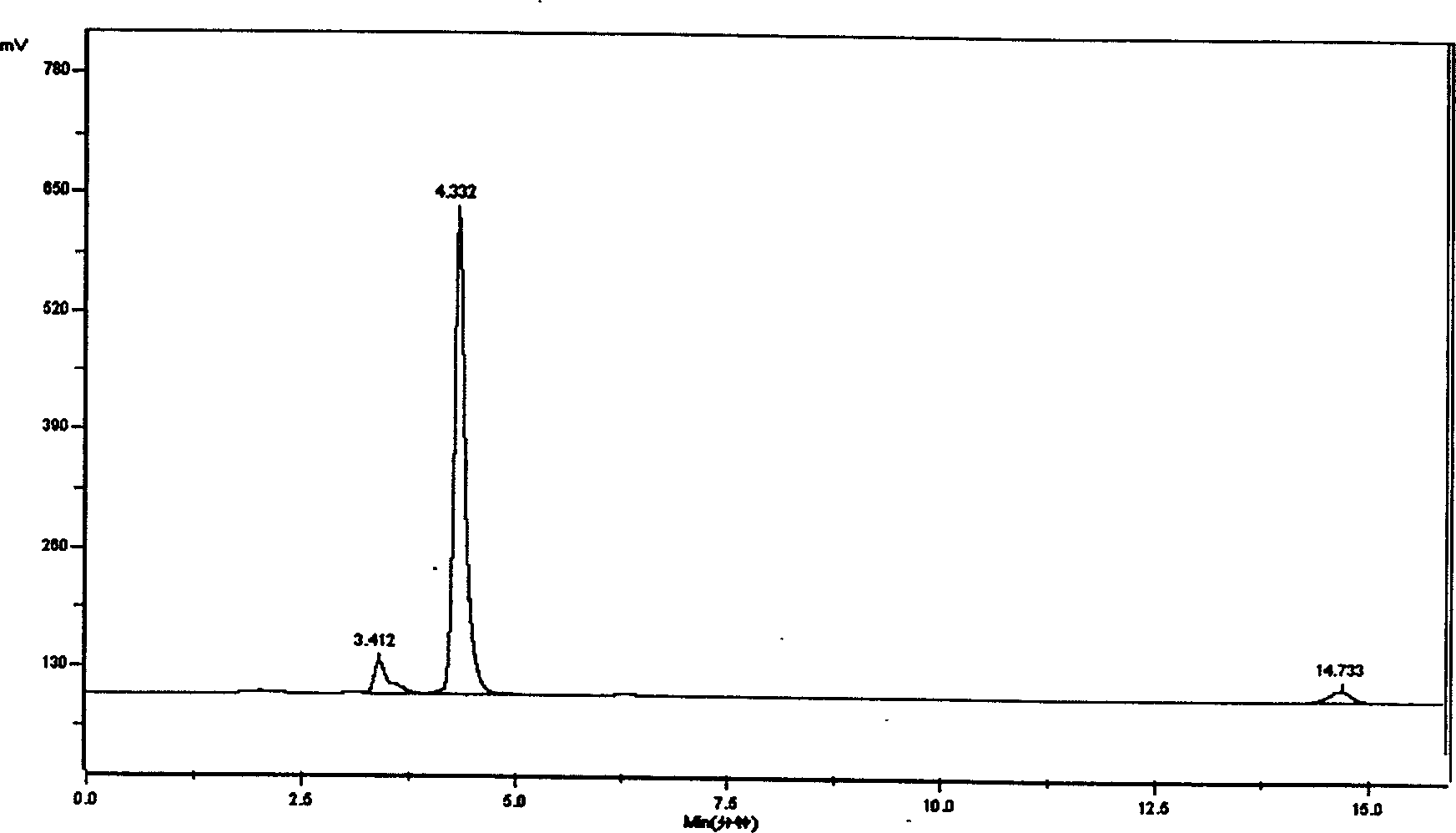
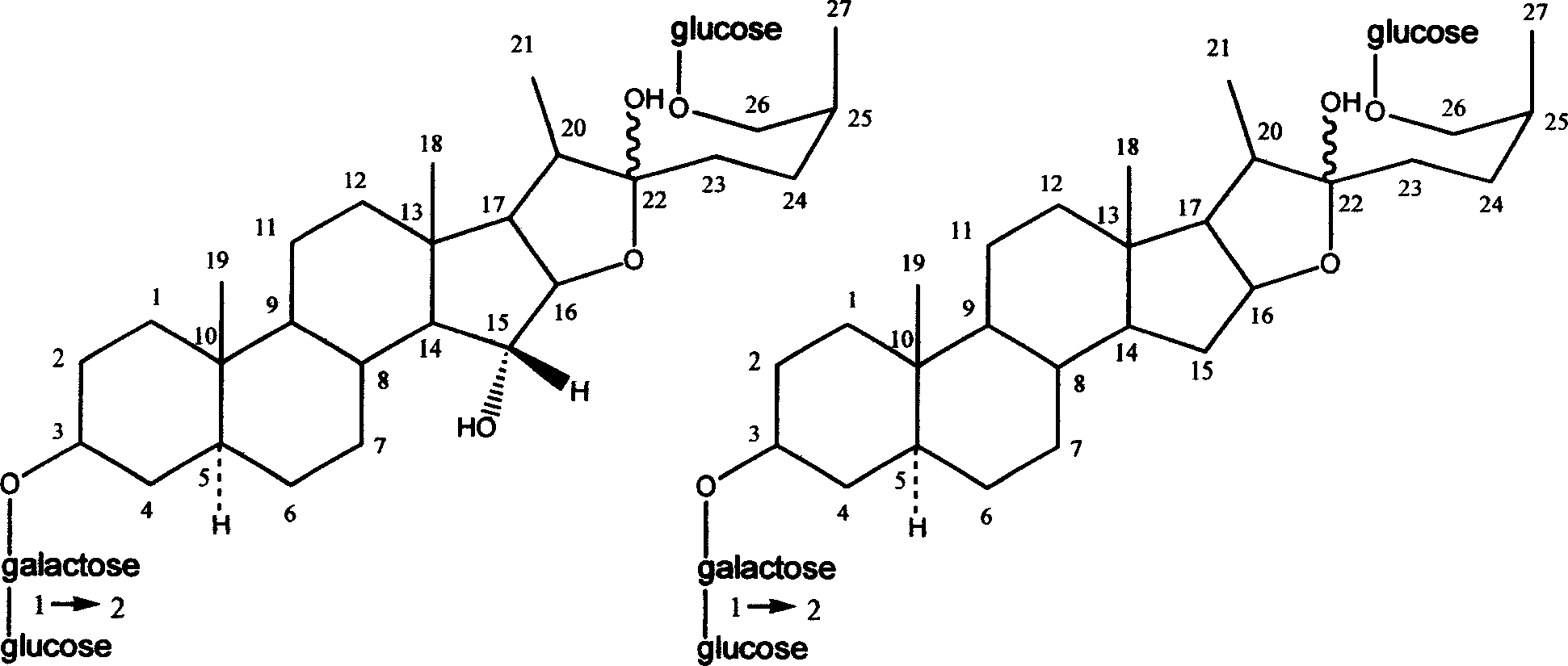
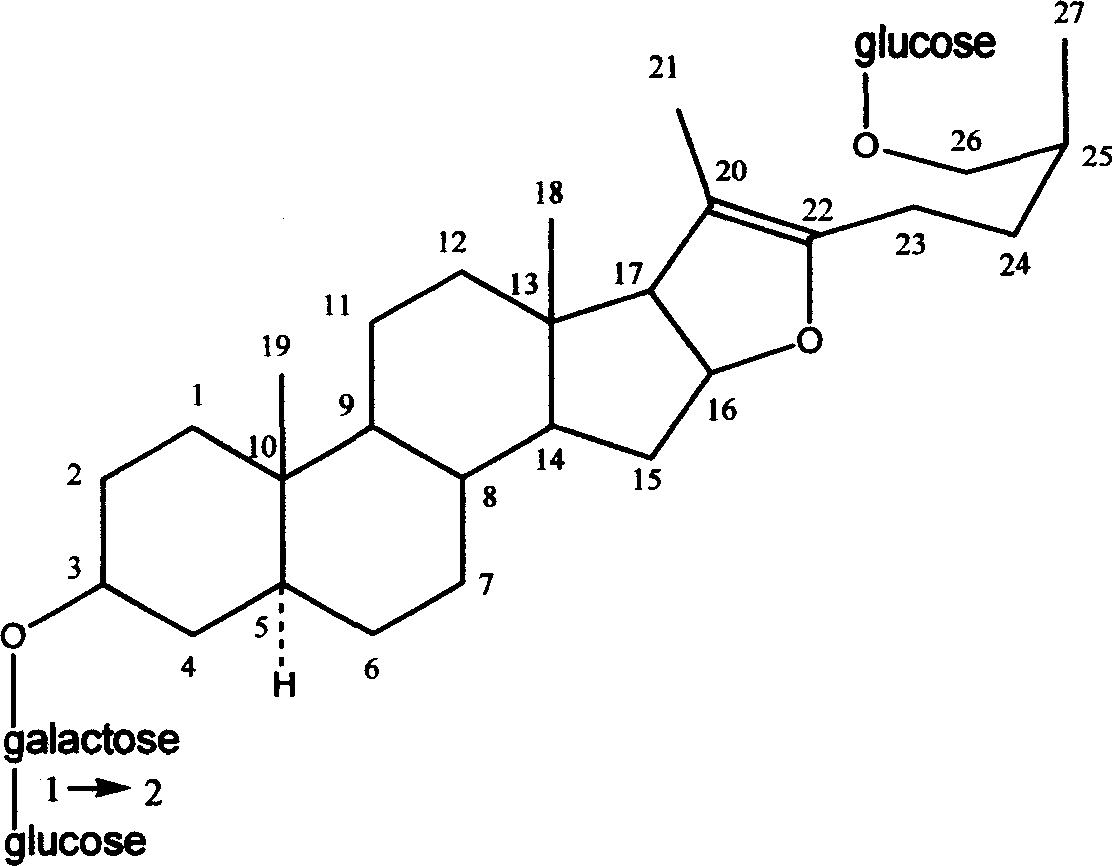
Injecta of anemarrhena extractive
A technology of Anemarrhena extract and injection, which is applied in the field of medicine and can solve the problems of low bioavailability, no visible and slow curative effect of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] The preparation of embodiment 1 Anemarrhena extract
[0105] Cut Anemarrhena into thin slices, take 10kg, infiltrate in 30L of 30% ethanol for 12 hours, extract by percolation, and collect 240L of percolation liquid. Concentrate the percolation liquid to 15L; filter the concentrated liquid, pass the filtrate into a chromatographic column equipped with 20L D101 resin, and successively elute with 300L of distilled water, 300L of 20% ethanol, and 120L of 60% ethanol, and collect 60% ethanol for elution Part; the 60% ethanol eluate was concentrated to 10L under reduced pressure, passed into a chromatographic column equipped with 20L D101 type resin; eluted with 20% ethanol 300L, 60% ethanol 120L successively, collected 60% ethanol eluted part, Concentrate under reduced pressure and dry to obtain light yellow crystalline powder, which is the Anemarrhena extract of the present invention, with a total weight of 0.14kg and a yield of 1.4% by crude drug weight.
Embodiment 2
[0106] The preparation of embodiment 2 Anemarrhena extract
[0107] Cut the medicinal material Anemarrhena into thin slices, take 10kg, heat extract with 50% ethanol 50L successively at 90-100°C for 3 times, each extraction time is 90 minutes, combine the extracts from 3 times, concentrate to 20L, filter, and pass the filtrate into In a chromatographic column equipped with 25L of ZTC-1 resin, elute with 500L of distilled water, 250L of 20% ethanol, and 150L of 50% ethanol in sequence, and collect the eluted part of 50% ethanol; concentrate the 50% ethanol eluate to 8L under reduced pressure , into a chromatographic column equipped with 10kg polyamide, elute with distilled water, collect 30L of distilled water eluate, concentrate under reduced pressure, and spray dry to obtain a light yellow powder, which is the Anemarrhena extract of the present invention, weighing 0.17kg in total, The yield is 1.7% by crude drug weight.
Embodiment 3
[0108] The preparation of embodiment 3 powder injections
[0109] Take 30g of Anemarrhena extract, add 30g of dextran, add 500ml of water for injection, stir to dissolve; add water for injection to 2000ml, add 3.0g of activated carbon for needles, stir for 30 minutes; decarbonize and filter; use 0.22μm microporous Membrane filtration; filling into sterile vials, 2ml per bottle, half stoppered; freeze-dried, then stoppered and capped.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com