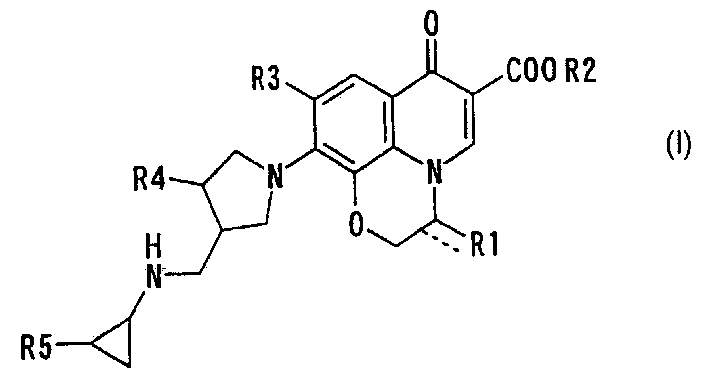
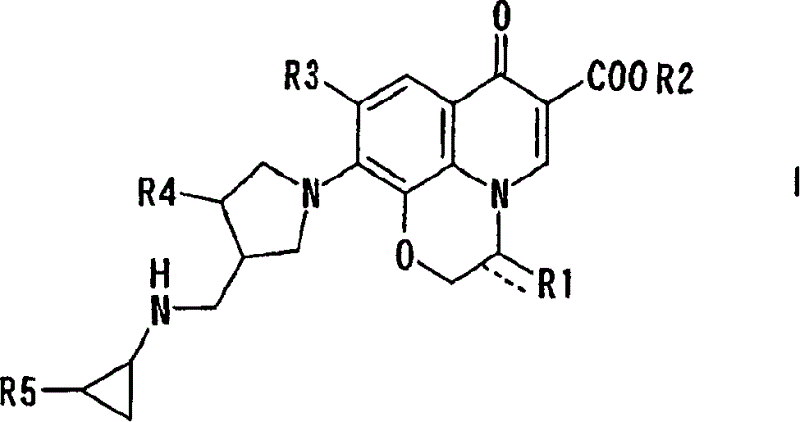
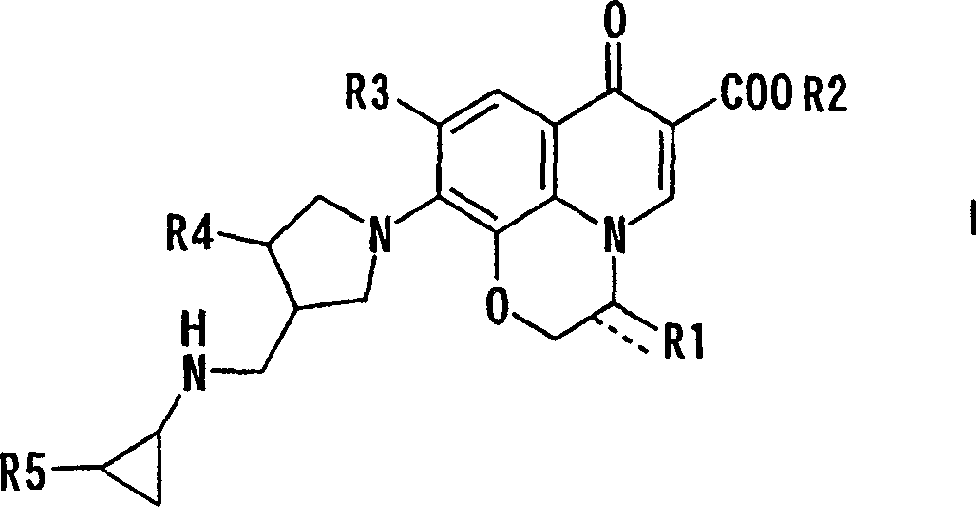
10-(3-cyclopropylaminomethyl-1-pyrrolidinyl)pyridobenzoxazinecarboxylic acid derivative effective against resistant bacterium
一种环丙基氨基甲基、苯并噁嗪的技术,应用在新型10-吡啶并苯并噁嗪羧酸衍生物及其盐和水合物领域,能够解决革兰氏阳性细菌抗菌活性弱、金黄色葡萄球菌治疗困难、未有记载耐性菌抗菌活性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0027] The following examples illustrate the test examples and preparation methods of the compounds of the present invention in detail.
reference example 1
[0029] Bis(acetate-O)[(3R)-9,10-difluoro-3-fluoromethyl-2,3-dihydro-7-oxo-7H-pyridine Pyrido[1,2,3-d,e][1,4]benzoxazine-6-carboxylate-O 6 ,O 7 ] boron
[0030] Boric acid (12.8 g) and acetic anhydride (63.4 g) were mixed, zinc chloride (236 mg) was added, and the mixture was stirred at room temperature for 0.5 hours. To this mixture was added (3R)-9,10-difluoro-3-fluoromethyl-2,3-dihydro-7-oxo-7H-pyrido[1,2,3-d,e][ 1,4] Ethyl benzoxazine-6-carboxylate (22.6 g), stirred at 60°C for 2.5 hours. The reaction solution was concentrated under reduced pressure, and the residue was dissolved in ethyl acetate (300 mL). The solution was washed with saturated aqueous sodium bicarbonate (2 x 200 mL), then water (100 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified with a silica gel column (dichloromethane: acetone = 7: 1), and the precipitated yellow amorphous was crystallized with a mixture of acetone-diethyl ether to ob...
reference example 2
[0036] Bis(acetate-O)[(3S)-9,10-difluoro-2,3-dihydro-3-methoxymethyl-7-oxo- 7H-pyrido[1,2,3-d,e][1,4]benzoxazine-6-carboxylate-O 6 ,O 7 ] Synthesis of boron
[0037] first step:
[0038] (3S)-9,10-Difluoro-2,3-dihydro-3-hydroxymethyl-7-oxo-7H-pyrido[1,2,3-d,e][1,4] Ethyl benzoxazine-6-carboxylate (1.30g) was suspended in anhydrous dimethyl sulfoxide (40mL), silver (I) oxide (4.63g) and methylated iodine (1.25mL) were added, and the Stir at room temperature for 21 hours. The insoluble matter in the reaction solution was filtered off, and the filtrate was concentrated under reduced pressure. The residue was purified with a silica gel column (dichloromethane:acetone=5:1) to obtain 740 mg of (3S)-9,10-difluoro-2,3-dihydro-3-methoxymethyl as a white powder -Ethyl 7-oxo-7H-pyrido[1,2,3-d,e][1,4]benzoxazine-6-carboxylate.
[0039] MS (EI) m / z: 339 (M + )
[0040] Elemental analysis value (%): C 16 h 15 f 2 NO 5
[0041] Calculated: C: 56.64, H: 4.46, N: 4.13
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com