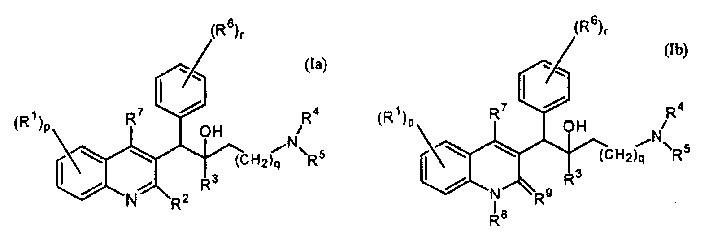
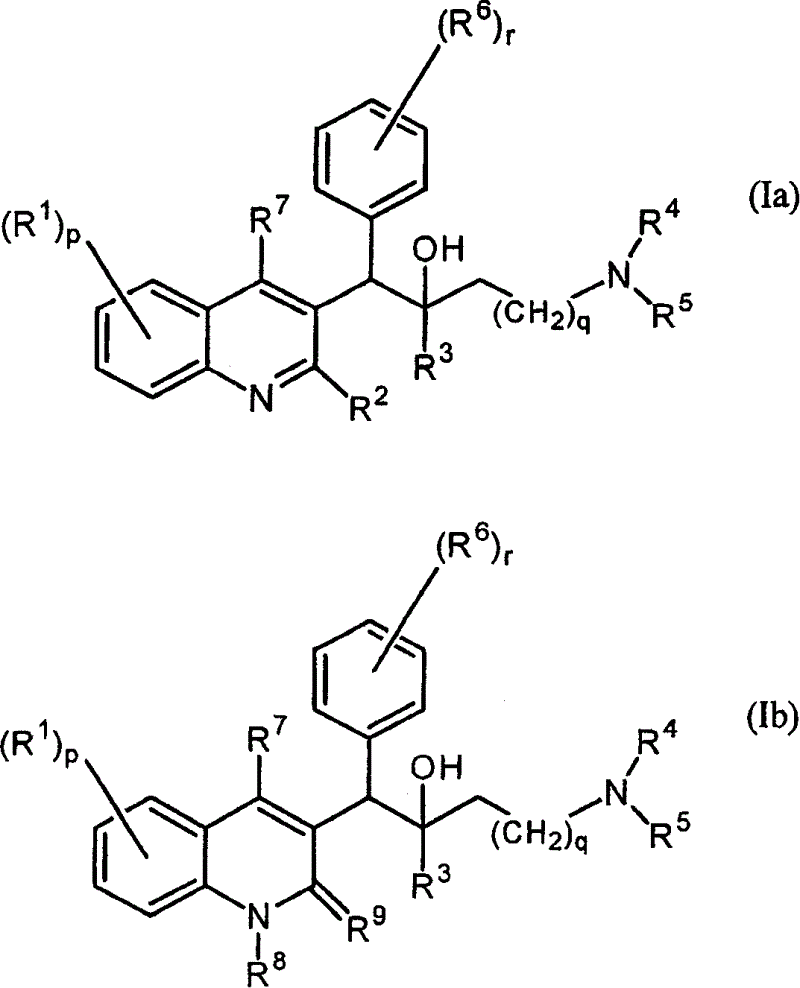
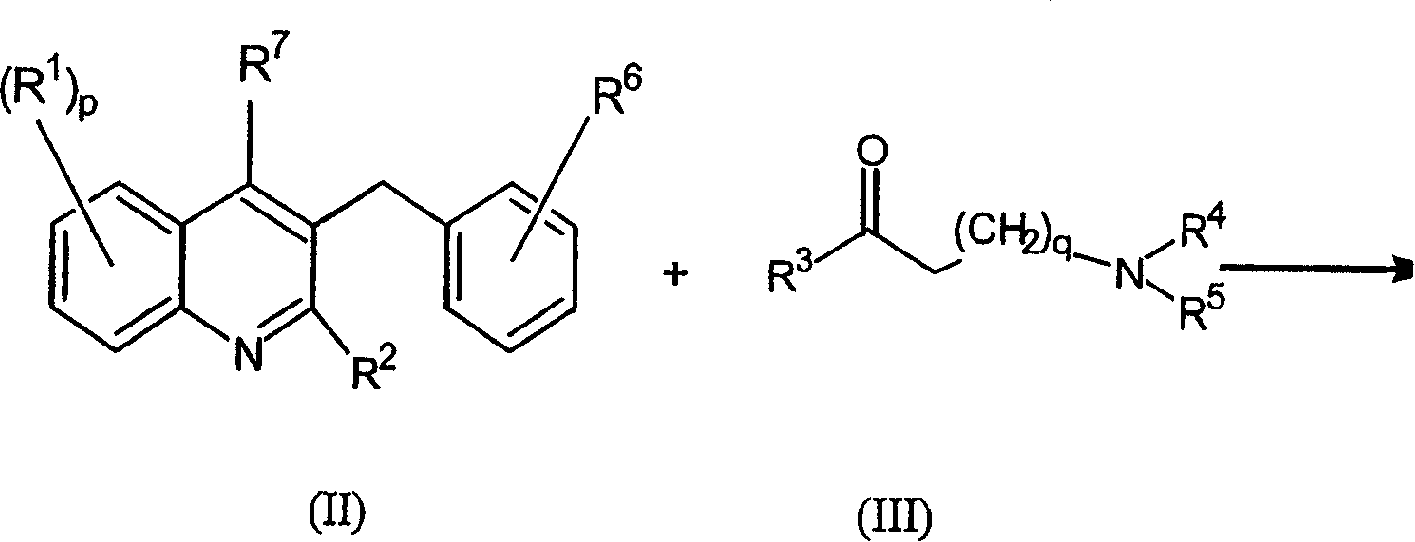
Quinoline derivatives and their use as mycobacterial inhibitors
一种化合物、药用酸的技术,应用在喹啉衍生物及其作为分枝杆菌抑制剂的应用领域,能够解决昂贵、药物有毒性、边缘等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A1
[0121] Preparation of intermediate compound 1
[0122]
[0123] Phenylpropionyl chloride (0.488mol) was added dropwise to 4-bromoaniline (0.407mol) at room temperature in Et 3 N (70ml) and CH 2 Cl 2 (700ml), and the mixture was stirred at room temperature overnight. This mixture was poured into water and concentrated NH 4 OH, and CH 2 Cl 2 extraction. The organic layer was dried (MgSO 4 ), filtered and the solvent was evaporated. The residue was crystallized from ether. The residue (119.67 g) was placed in CH 2 Cl 2 medium and washed with HCl 1N. The organic layer was dried (MgSO 4 ), filtered and the solvent was evaporated. Yield: 107.67 g of intermediate compound 1.
[0124] Preparation of intermediate compound 9
[0125]
[0126] Thus, using the same procedure as intermediate compound 1, but employing 4-methylphenylpropionyl chloride, intermediate compound 9 was prepared.
Embodiment A2
[0128] Preparation of intermediate compound 2
[0129]
[0130] This reaction was performed 2 times. POCl at 10°C 3 (1.225mol) was added dropwise to DMF (0.525mol). Intermediate compound 1 (prepared according to Al) (0.175 mol) was then added at room temperature. The mixture was stirred overnight at 80 °C, poured onto ice, and washed with CH 2 Cl 2 extraction. The organic layer was dried (MgSO 4 ), filtered and the solvent was evaporated. The product was used without further purification. Yield: (77.62 g; Yield = 67%).
[0131] Preparation of intermediate compound 10
[0132]
[0133] Therefore, according to the same method as intermediate compound 2, intermediate compound 10 was prepared from intermediate compound 9 (prepared according to A1).
Embodiment A3
[0135] Preparation of intermediate compound 3
[0136]
[0137] Intermediate compound 2 (prepared according to A2) (0.233 mol) in CH 3 A solution of ONa (30%) in methanol (222.32ml) and a mixture in methanol (776ml) were stirred and refluxed overnight, then poured onto ice and washed with CH 2 Cl 2 extraction. The organic layer was separated and dried (MgSO 4 ), filtered and the solvent was evaporated. The residue was purified by silica gel column chromatography (eluent: CH 2 Cl 2 / cyclohexane 20 / 80, then 100 / 0; 20-45 μm). Pure fractions were collected and the solvent was evaporated. Yield: 25 g of intermediate compound 3 (yield = 33%; mp. 84°C) as a white powder.
[0138] Preparation of intermediate compound 11
[0139]
[0140] Thus, according to the same method as intermediate compound 3, intermediate compound 11 was prepared from intermediate compound 10 (prepared according to A2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com