Methods of preparing aqueous colored pigment dispersions, and inkjet ink compositions
A technology of colored pigments and inkjet inks, applied in the direction of replication/marking methods, coupling reaction of azo dyes, inks, etc., can solve the problem of pigments not easy to disperse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] The present invention relates to a method for preparing an aqueous colored pigment dispersion and an inkjet ink composition.
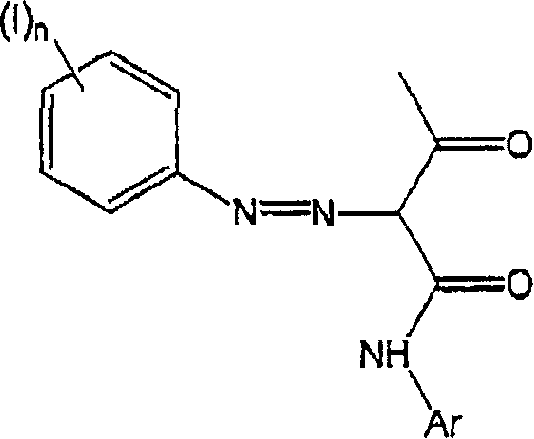
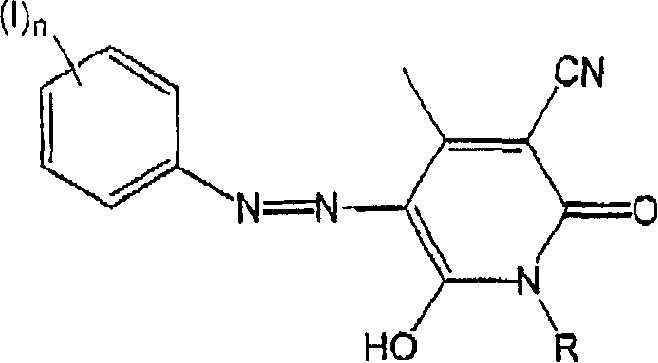
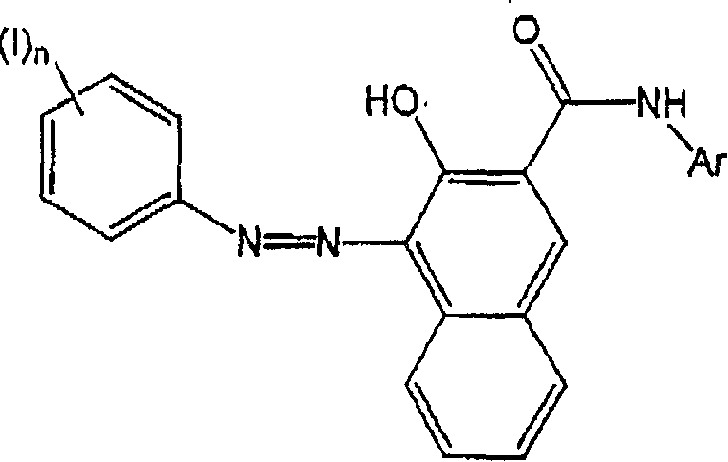
[0015] The method of the present invention comprises the steps of combining a) a colored pigment, b) an azo coupler, c) an aromatic amine, d) a diazotizing agent, and e) an aqueous medium. Each of these components will be described in detail below. These components may be combined in any order and under any conditions to produce an aqueous colored pigment dispersion. For example, the color pigment and azo coupler can be combined, dry or in a liquid medium, to form a pretreated color pigment, which is subsequently combined with an aromatic amine and a diazotizing agent, preferably in an aqueous medium. Furthermore, the aromatic amine and the diazotizing agent may be combined in a liquid medium, preferably an aqueous medium, to form the diazonium salt. This can then be combined with the color pigments and / or azo couplers described above, or comb...
Embodiment 1
[0063] A rotor-stator high shear mixer (Silverson L4RT-A) was equipped with a 4 liter stainless steel beaker and the mixer was immersed in an ice bath. About 75 g of Pigment Yellow 74 and 1000 g of water were placed in a beaker and the mixture was homogenized at 7200 rpm for 15 minutes. A solution of 2.07 g (0.01 mol) of acetoacetyl-o-methoxybenzylamide in 20 mL of isopropanol was added thereto, and the mixture was stirred for a further 15 minutes.
[0064] In a separate vessel, 4.35 g (0.025 mol) of sulfanilic acid was mixed with 30 ml of 1N HCl and 1.73 g (0.025 mol) of sodium nitrite at 5-10°C to form the corresponding diazonium salt. This was then added to the mixture of Pigment Yellow 74 and acetoacetyl-o-methoxybenzylamide with stirring. Keep the temperature at about 10°C. The mixture was adjusted to pH 5-6 by dropwise addition of 5M sodium hydroxide solution. Mixing was continued for an additional 2 hours. By removing a small portion of the mixture and adding it in ...
Embodiment 2
[0068] A rotor-stator high shear mixer (Silverson L4RT-A) was equipped with a 4 liter stainless steel beaker and the mixer was immersed in an ice bath. About 75 g of Pigment Yellow 74 and 1000 g of water were placed in a beaker and the mixture was homogenized at 7200 rpm for 15 minutes. Then add 3.85g of compound I shown below (at pH 4.5-6.0, using a diazonium salt and an azo coupler in a molar ratio of 1:1, by mixing the diazonium salt of sulfanilic acid with acetoacetyl-o-methoxy prepared by azo coupling) in 200 mL of water. The mixture was then stirred for a further 2 hours.
[0069]
[0070] Compound I
[0071] The mixture was transferred to a Telsonic flow-through sonicator and sonicated for 2 hours to prepare an aqueous color pigment dispersion. The resulting yellow pigment dispersion was purified using a 50 nm diafiltration membrane column and concentrated to 10% solids. The median particle size of the bright yellow dispersion is about 150 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com