Novel compounds and compositions as protein kinase inhibitors
A compound, alkyl technology, applied in the field of new compounds and compositions as protein kinase inhibitors, can solve the problem of drug resistance of STI-571
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] B. Preparation of compounds
[0066] The invention also includes processes for the preparation of the compounds of the invention. In the reactions described, when reactive functional groups such as hydroxyl, amino, imino, mercapto or carboxyl are desired in the final product, it may be necessary to protect these groups from their participation in the reaction. Conventional protecting groups can be used according to standard operating procedures, see, eg, T.W. Greene and P.G.M. Wuts, "Protective Groups in Organic Chemistry", John Wiley and Sons, 1991.
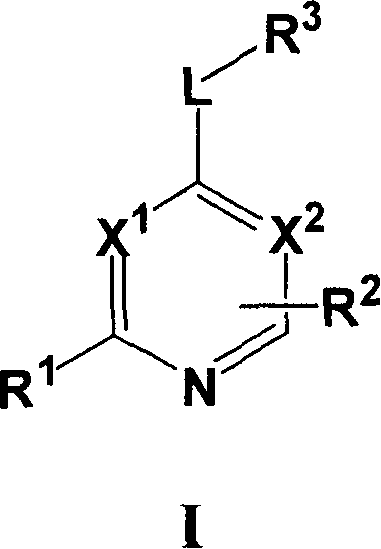
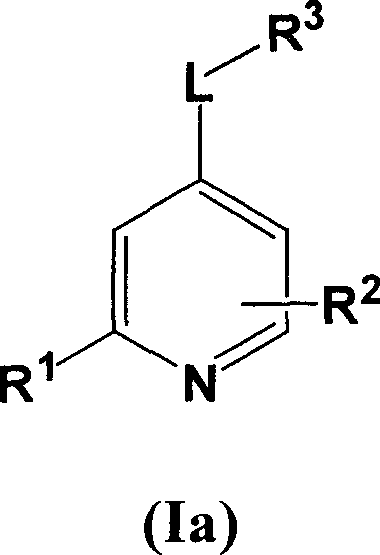
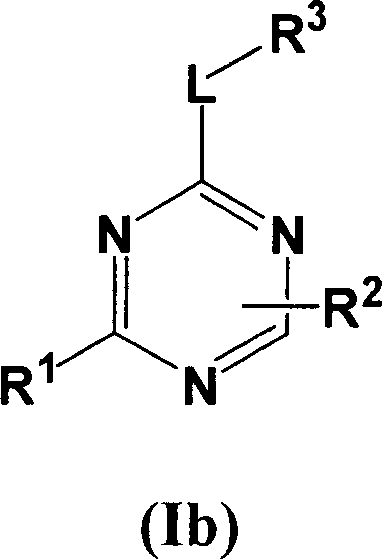
[0067] Compounds of formula I wherein L is a bond can be prepared using the procedure in Reaction Scheme I below:
[0068] Reaction Flow Diagram I
[0069]
[0070] where X 1 、X 2 , R 1 , R 2 and R 3 The definition of is as described above for formula I, Q represents a halogen group, such as iodine or chlorine, preferably chlorine.
[0071] Compounds of formula I can be prepared by reacting ...
reference example 1
[0135] Reference Example 1. (6-Chloro-pyrimidin-4-yl)-(4-trifluoromethoxy-phenyl)-amine
[0136]
[0137] 1.0 g of 4,6-dichloropyrimidine (6.7 mmol) and 1.2 g of p-trifluoromethoxyaniline (6.7 mmol) were dissolved together in 15 mL of ethanol, and then 1.75 mL of DIEA (10 mmol) was added thereto. The reaction was carried out at reflux for 2 hours, then it was cooled to room temperature. After evaporating off the solvent, the crude product was purified by flash chromatography (EA / hexane=3:7) to obtain 1.94 g of (6-chloro-pyrimidin-4-yl)-(4-trifluoromethoxy base-phenyl)-amine.
reference example 2
[0138] Reference Example 2.4-[6-(4-Trifluoromethoxy-phenylamino)-pyrimidin-4-yl]-benzoic acid
[0139]
[0140] 200 mg (4-chloro-pyrimidin-6-yl)-(4-trifluoromethoxy-phenyl)-amine (0.69 mmol) prepared in Reference Example 1 was added to 115 mg of 4-carboxyphenylboronic acid (0.69 mmol), 40 mg tetrakis(triphenylphosphine) palladium (0.034 mmol) and 292 mg sodium carbonate (2.76 mmol) in a flask. To this flask was added 10 mL of solvent MeCN / H 2 O(1:1). After filling with argon, the flask was heated to 90° C. for 8 hours. The hot reaction solution was filtered and collected. To this solution was added 6N HCl solution until its pH was below 5. The pale solid 4-[6-(4-trifluoromethoxy-phenylamino)-pyrimidin-4-yl]-benzoic acid (220 mg) was collected by filtration and washed twice with 5 mL of water .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com