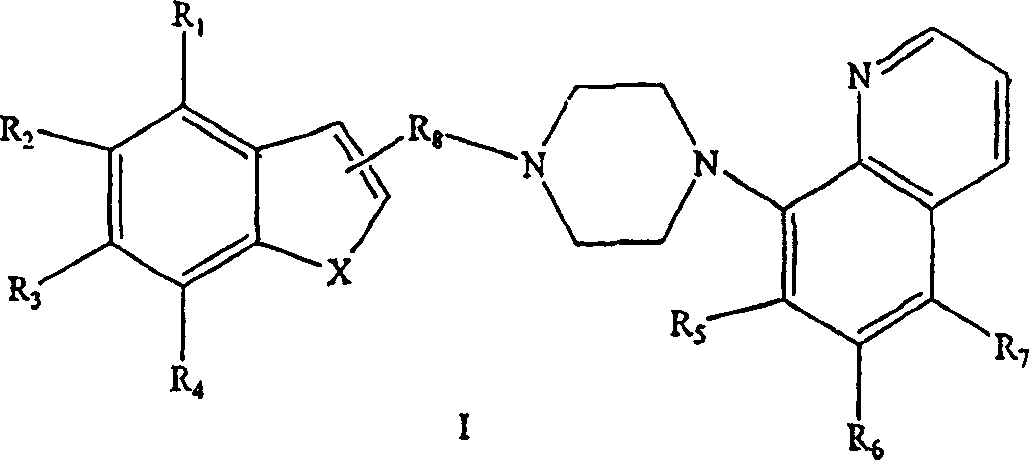
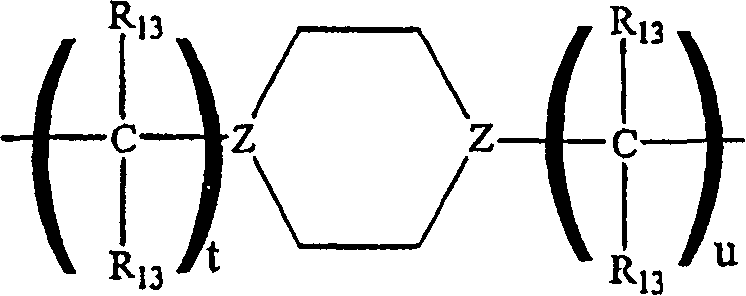
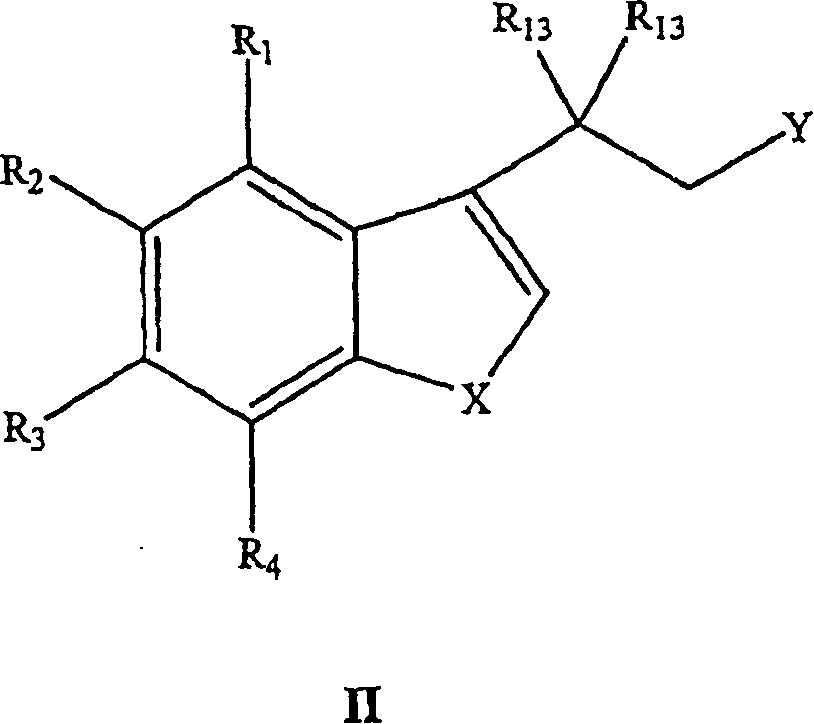
Benzofuranyl-and benzothienyl-piperazinyl quinolines as 5-serotonin reuptake inhibitors
A technology based on benzofuran and piperazine, which is applied in medical preparations containing active ingredients, organic active ingredients, nervous system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0230] Embodiment 1: Intermediate 1--8-piperazino quinoline
[0231] This intermediate can generally be prepared as described in WO 00 / 40554, which is incorporated herein by reference.
Embodiment 2
[0232] Example 2: Intermediate 2--6-fluoro-8-bromo-quinoline
[0233]
[0234] To a mixture of 7.0 g of 2-bromo-4-fluoro-aniline, 7.0 g of glycerol and 13.0 g of m-nitrobenzenesulfonic acid sodium salt, 20 ml of 70% sulfuric acid was added dropwise. The reaction temperature was raised to 150°C for 4 hours. The resulting mixture was cooled, poured into water, neutralized with NaOH, and the precipitate formed was filtered to yield 34.7 g of 6-fluoro-8-bromo-quinoline. MS (ES) m / z (relative intensity): 227 (M + +H, 100).
Embodiment 3
[0235] Example 3: Intermediate 3--6-fluoro-8-(t-Boc)-piperazino-quinoline
[0236]
[0237] To a mixture of 2.2 g of 6-fluoro-8-bromo-quinoline in THF was added 0.045 g of Pd 2 (dba) 3, 1.3g NaOt-Bu, 0.044g binap, 0.052g tetrakis(triphenylphosphine)palladium(0) and 2.2g t-Boc piperazine. The resulting mixture was refluxed for 3 hours. The reaction mixture was then cooled to room temperature, diluted with ether, filtered through celite and concentrated in vacuo. The crude product was purified by flash chromatography to afford 3.0 g of 6-fluoro-8-(t-Boc)-piperazino-quinoline. MS (ES) m / z (relative intensity): 332 (M + +H, 100).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com