Use of hydrophobic-interaction-chromatography or hinge-region modifications for the production of homogeneous antibody-solutions
A hinge, antibody molecule technology, applied in the direction of peptides, immunoglobulins, chemical instruments and methods, etc., can solve the problems of negative impact on overall yield, increased effort required for downstream processing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0315] Preparations of dosage forms for parenteral administration include sterile aqueous or non-aqueous solutions, suspensions and emulsions. Examples of non-aqueous solvents are propylene glycol, polyethylene glycol, vegetable oils such as olive oil, and injectable organic esters such as ethyl oleate. Aqueous carriers include water, ethanol / water solutions, emulsions or suspensions, including saline and buffered media. In the present invention, the pharmaceutically acceptable carrier includes but not limited to: 0.01-0.1M and preferably 0.05M phosphate buffer saline or 0.8% saline. Other common parenteral vehicles include phosphate solutions, Ringer's dextrose, dextrose and sodium chloride, lactated Ringer's, or fixed oils. Intravenous vehicles include fluids, nutrient replenishers, electrolyte replenishers such as those based on Ringer's dextrose, and the like. There may also be preservatives and other additives such as antimicrobials, antioxidants, chelating agents, and ...
Embodiment 1
[0361] Example 1: Identification of A and B isoforms
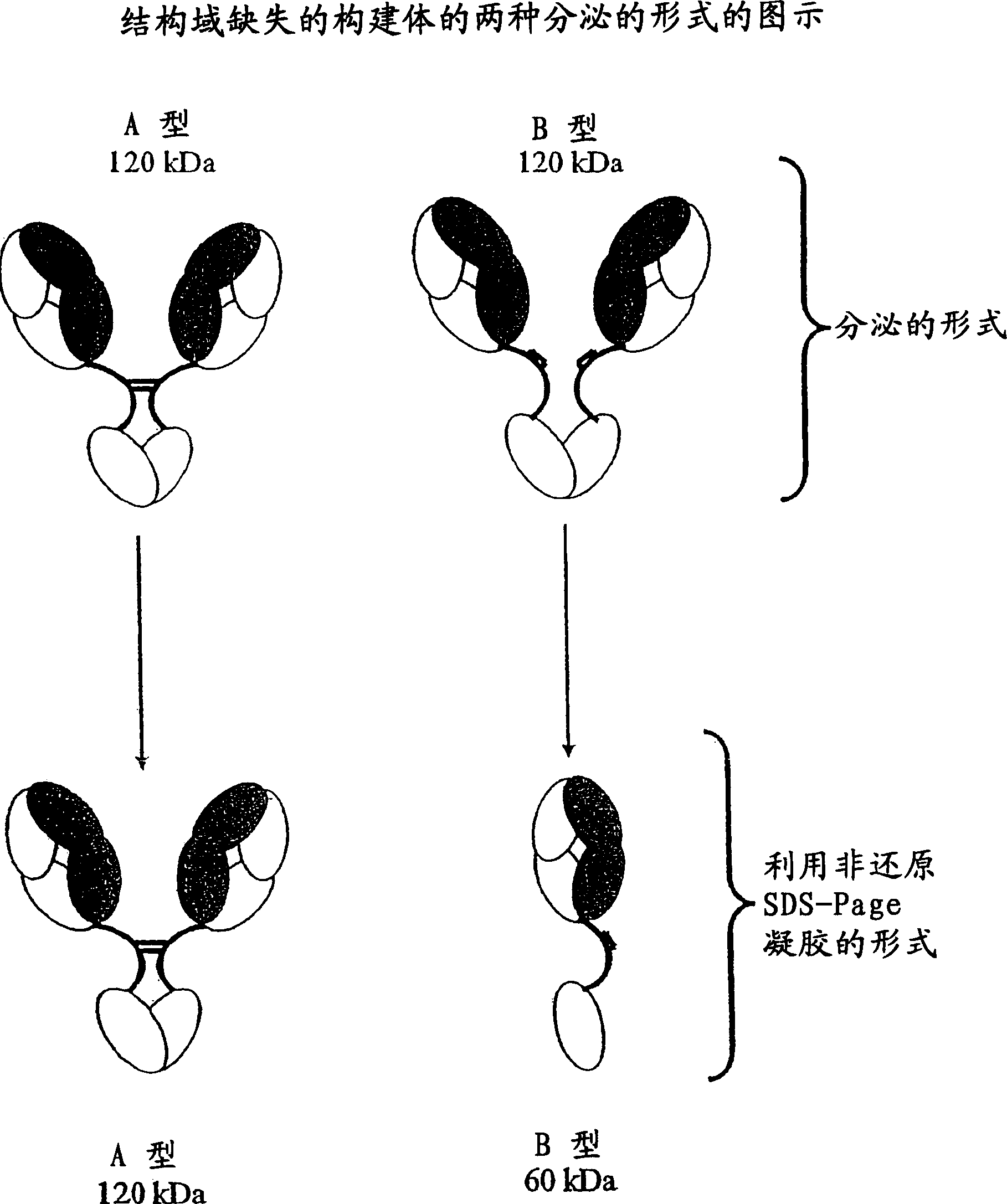
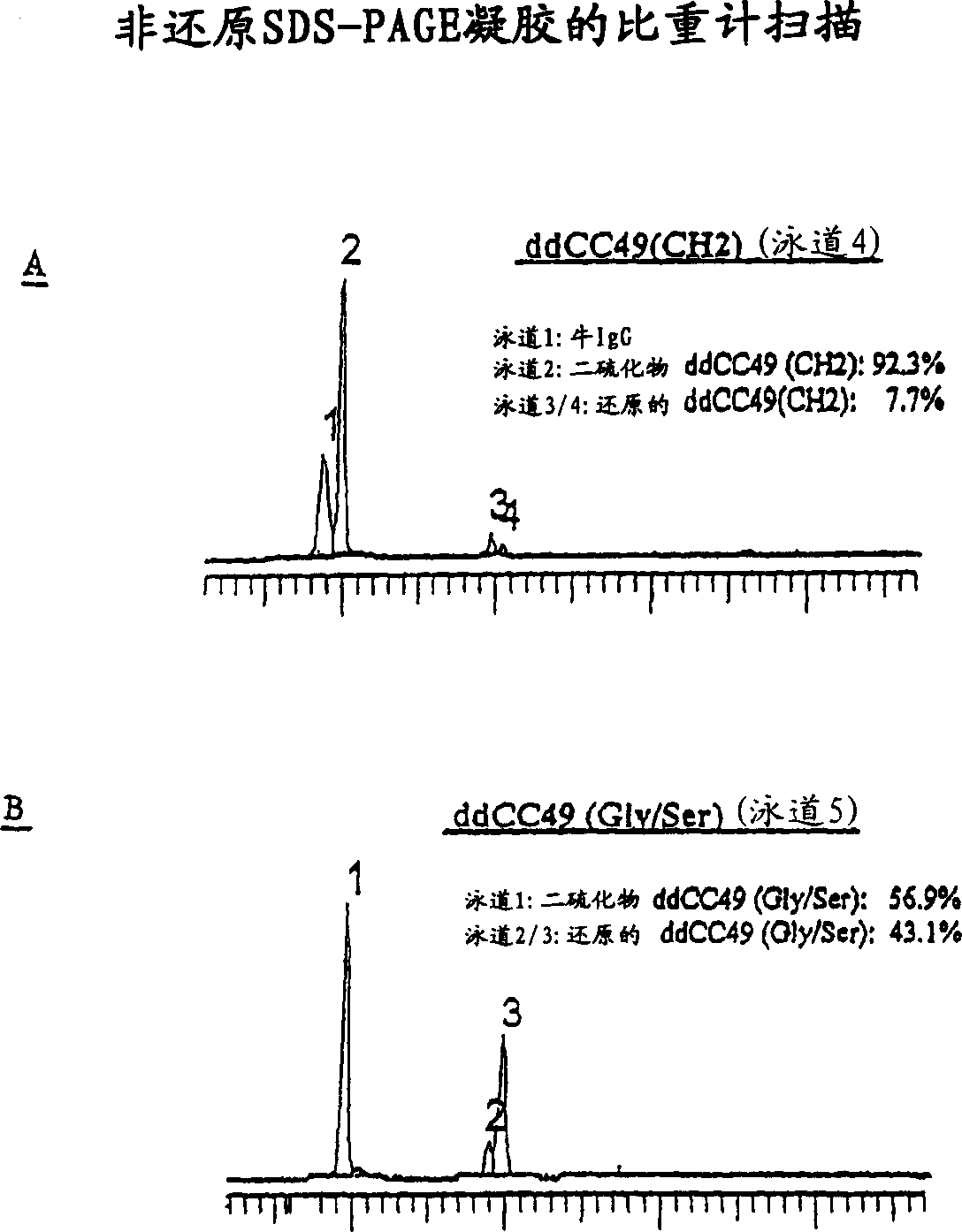
[0362] The solution of antibody molecules contains two different isotypes. One of these, type A, comprises heavy chain molecules linked via at least one disulfide bond. Another type, B, comprises heavy chain molecules that are not linked via at least one disulfide bond. In intact gamma 1 MAbs, such as Rituxan(R), type B is absent or very infrequent. However, the frequency of type B is much higher compared to domain deleted (dd) constructs with similar hinges. These forms can be differentiated using denatured non-reduced SDS page. In domain-deleted antibody preparations, type A is a 120 kDa dimer and type B is a 60 kDa monomer ( figure 1 ). figure 2 A and 2B show densitometer plots of non-reducing SDS-PAGE gels of ddCC49(CH2) and ddCC49(Gly / Ser), respectively.
Embodiment 2
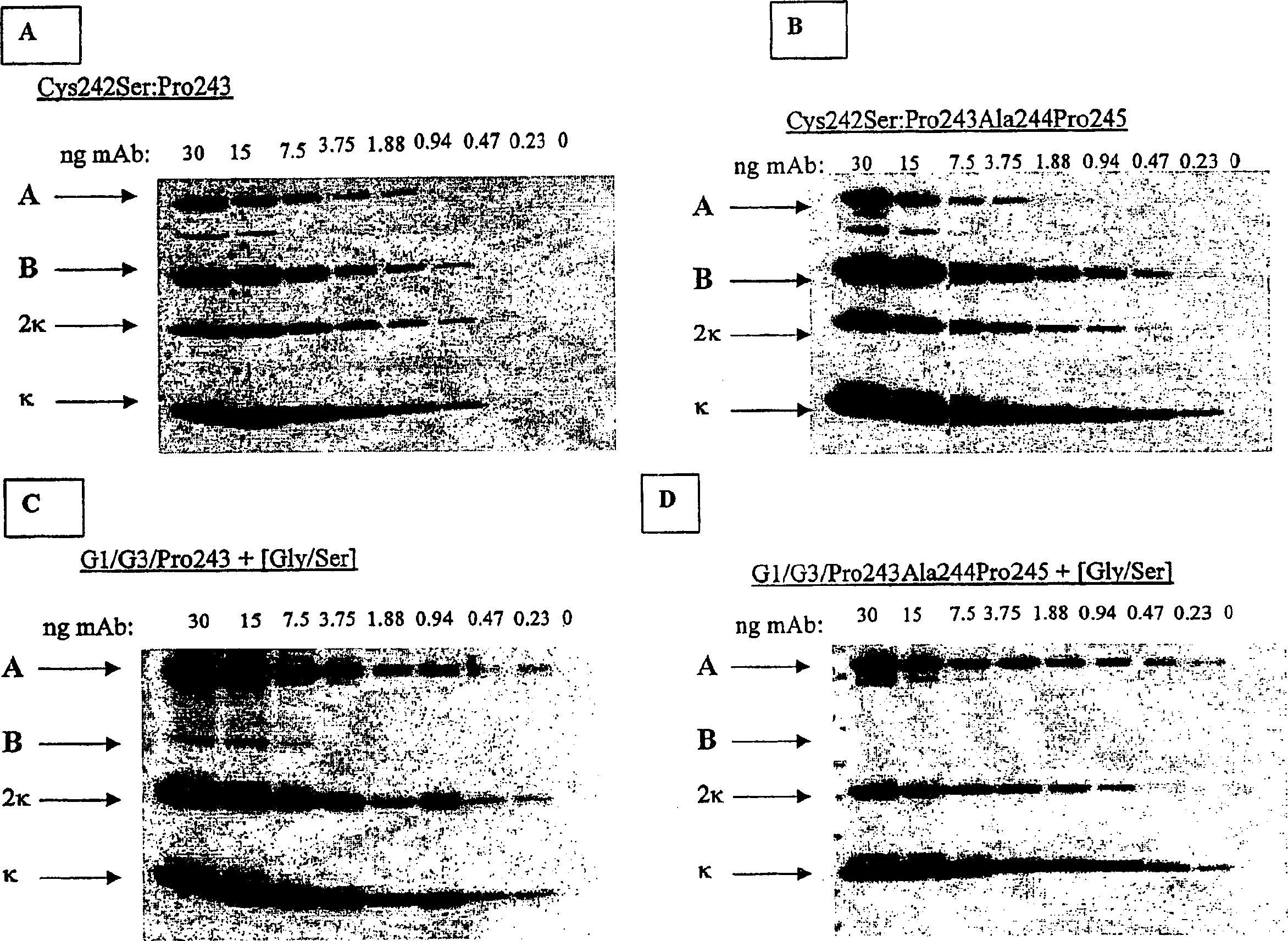
[0363] Example 2: Identification of hinge region heterology in CH2 domain-deleted Mab fragments
[0364]The hinge domain can be subdivided into three distinct regions: upper, middle, and lower hinge regions (Roux et al. J. Immunol. 1998 161:4083). The polypeptide sequences comprising these regions are shown in Table 1 for the IgG1 and IgG3 hinges. In addition to two conserved cysteine residues, the IgG3 hinge region also contains a 15 amino acid motif repeated three times. Amino acid sequences from these regions were used to design synthetic IgG1 / IgG3 linker peptides. These consist of the IgG1 upper hinge residues corresponding to positions 226-238, the IgG1 middle hinge corresponding to positions 239-241, and a single IgG3 middle hinge repeat motif corresponding to positions 241EE-242, which binds position 243 or added proline at positions 243, 244, and 245 (Kabat numbering system), respectively, alanine, proline, followed by a flexible Gly / Ser spacer (Table 2). In addit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap