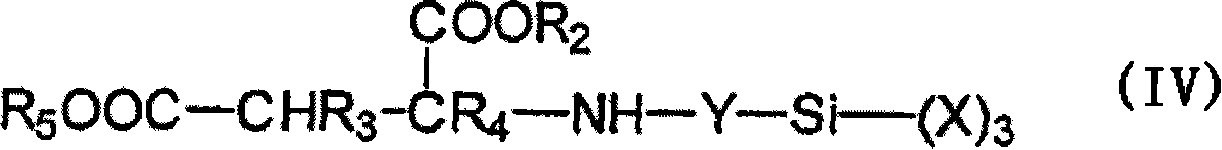
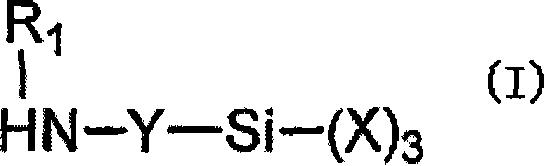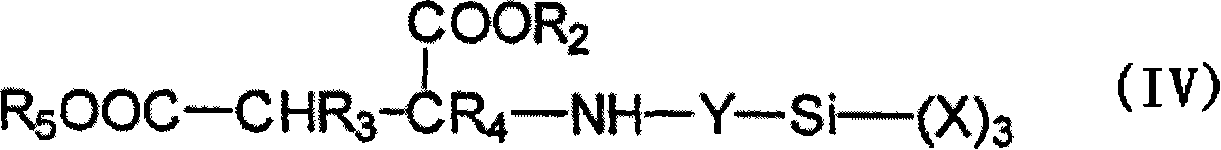Moisture-curable, polyether urethanes with reactive silane groups and their use as sealants, adhesives and coatings
一种聚醚氨酯、湿固化的技术,应用在聚脲/聚氨酯粘合剂、粘合剂类型、胶粘剂等方向,能够解决没有认识到活性硅烷基必要性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0097] The following raw material components were used in the examples:
[0098] Preparation of silane-functionalized aspartate (SFA 1)
[0099] Aspartate resins were prepared according to US Patent 4,364,955. 1483 grams (8.27 equivalents) of 3-aminopropyltrimethoxysilane (Silquest A-1110, available from OSI Corporation) were added to a 5-liter flask equipped with a stirrer, thermocouple, nitrogen inlet and addition funnel, and condenser. ). Over two hours, 1423.2 grams (8.27 eq.) of diethyl maleate were added via the addition funnel. The temperature of the reactor was maintained at 25°C during this addition. The reactor was maintained at 25°C for an additional 5 hours, then the product was poured into a glass container and sealed under nitrogen. After one week, the unsaturation value was 0.6, indicating that the reaction was -99% complete.
[0100] Y-9669
[0101] N-Phenylaminopropyltrimethoxysilane (commercially available as A-9669 from OSI Corporation)
[0102] A-111...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
| degree of unsaturation | aaaaa | aaaaa |
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com