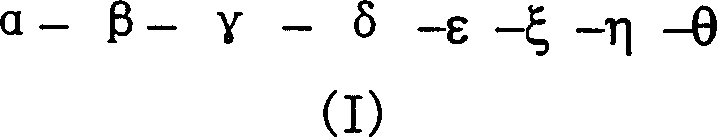Corticotropin releasing factor 2 receptor agonists
A variant, X21 technology, applied in the field of corticotropin-releasing factor 2 receptor agonists, which can solve problems such as skeletal muscle atrophy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0132] In the preparation of peptides, recombinant DNA technology or cell lines expressing these peptides can also be used. These recombination methods are well known in the art. Methods of generating rDNA molecules are well known in the art, see Sambrook et al., Molecular Cloning-A Laboratory Manual, Cold Spring Harbor Laboratory Press (1989). In order to express the recombinant peptide of the present invention, an expression vector is prepared, which includes a nucleic acid encoding a related polypeptide under the control of one or more regulatory elements. The nucleic acid sequence encoding the peptide of the invention can be deduced from the peptide sequence described or claimed herein.
[0133] By methods well known in the art, the isolated nucleic acid molecules encoding related peptides can be ligated into a suitable expression vector. The host expression vector system that can be used for the purpose of the present invention includes, but is not limited to: microorganisms,...
Embodiment 1
[0195] Savagine and other non-selective CRFR agonists are generally not effective in treating CRF 2 R-modulated conditions, because these agonists also activate CRF 1 R, which leads to undesirable side effects.
[0196] Table 2 lists the human urocortin I (hUcnI), human urocortin II (hUroII), human urocortin III (hUroIII), and human adrenocorticotropic hormone called SEQ ID NOs: 2, 4, 6, 8, 10, and 11, respectively. Comparison of CRF binding of natural sequence fragments of release factor (hCRF), ovine corticotropin (oCRF) and savagine (Svg).
[0197] SEQ ID
Embodiment 2
[0199] Table 3 lists the comparative CRF combinations of different embodiments of the present invention.
[0200] SEQ ID NO
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com