Retinoic acid-containing remedy for diabetes
A technology for retinoic acid and diabetes, applied in the field of anti-diabetic drugs, can solve the problems of diabetic coma, inability to adequately control blood sugar levels, etc., and achieve the effect of improving functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of Retinoic Acid Pharmaceutical Preparations
[0050] As retinoic acid, commercially available retinoic acid (solid particles) was used as it was.
[0051] (1) Preparation of retinoic acid-inorganic substance composite particles
[0052] 13.6 mg of retinoic acid was dissolved in 900 μL of ethanol (or methanol), and 100 μL of 0.5 N NaOH aqueous solution was added to the solution. At this point, the pH is 7-7.5. As a mother solution, 100 µL of this solution was collected, added to 100 µL of distilled water containing Tween 80 (trade name), and the mixture was stirred well. After about 30 minutes, a 5M aqueous solution of calcium chloride was added and the mixture was stirred. After 30 minutes, an aqueous solution containing 1M sodium carbonate was added, and the mixture was further stirred. After continuous stirring for 24 hours, the resulting solution was freeze-dried overnight to obtain the desired retinoic acid-calcium carbonate composite particles.
...
Embodiment 2
[0057] The injection solution prepared in Example 1 (containing 6 mg of retinoic acid) was subcutaneously injected into the back of each diabetic rat at a dose of 1 mL through a syringe equipped with a 18-21 gauge needle. When using an 18-gauge needle, the injection site should be closed with surgical Aron Alpha (trade name) after injection to prevent leakage of the drug solution.
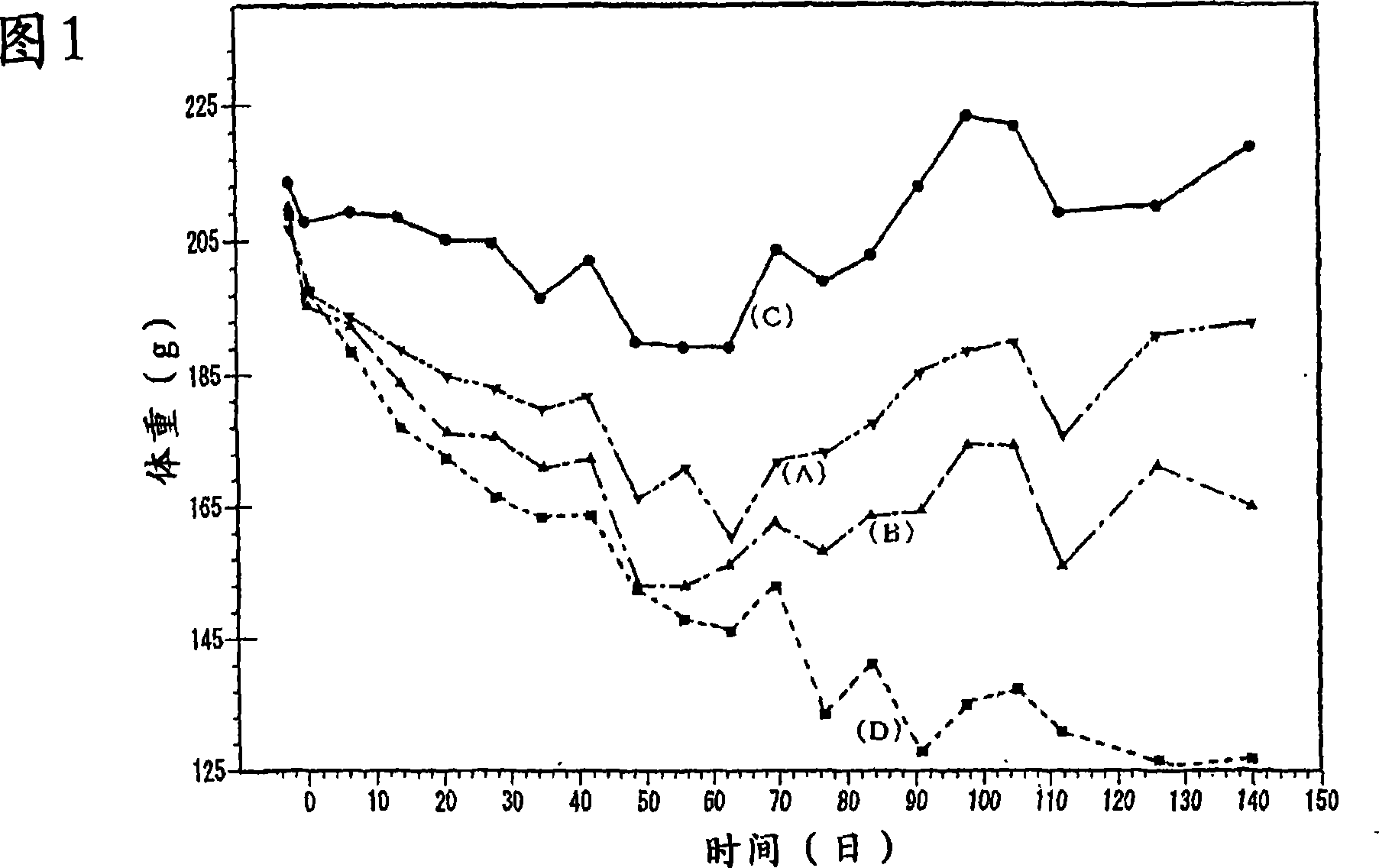
[0058] Subsequently, the change in body weight of each rat was observed, and the results are shown in FIG. 1 . As can be seen from Figure 1, the body weight of the control rats (D) not administered retinoic acid gradually decreased. Meanwhile, in the retinoic acid-administered rats (A) to (C), a decrease in body weight was observed at the initial stage, but a tendency to increase body weight was observed thereafter. In particular, the body weight of rats (C) administered with the pharmaceutical preparation of retinoic acid-organic substance composite particles decreased in a short period of time a...
Embodiment 3
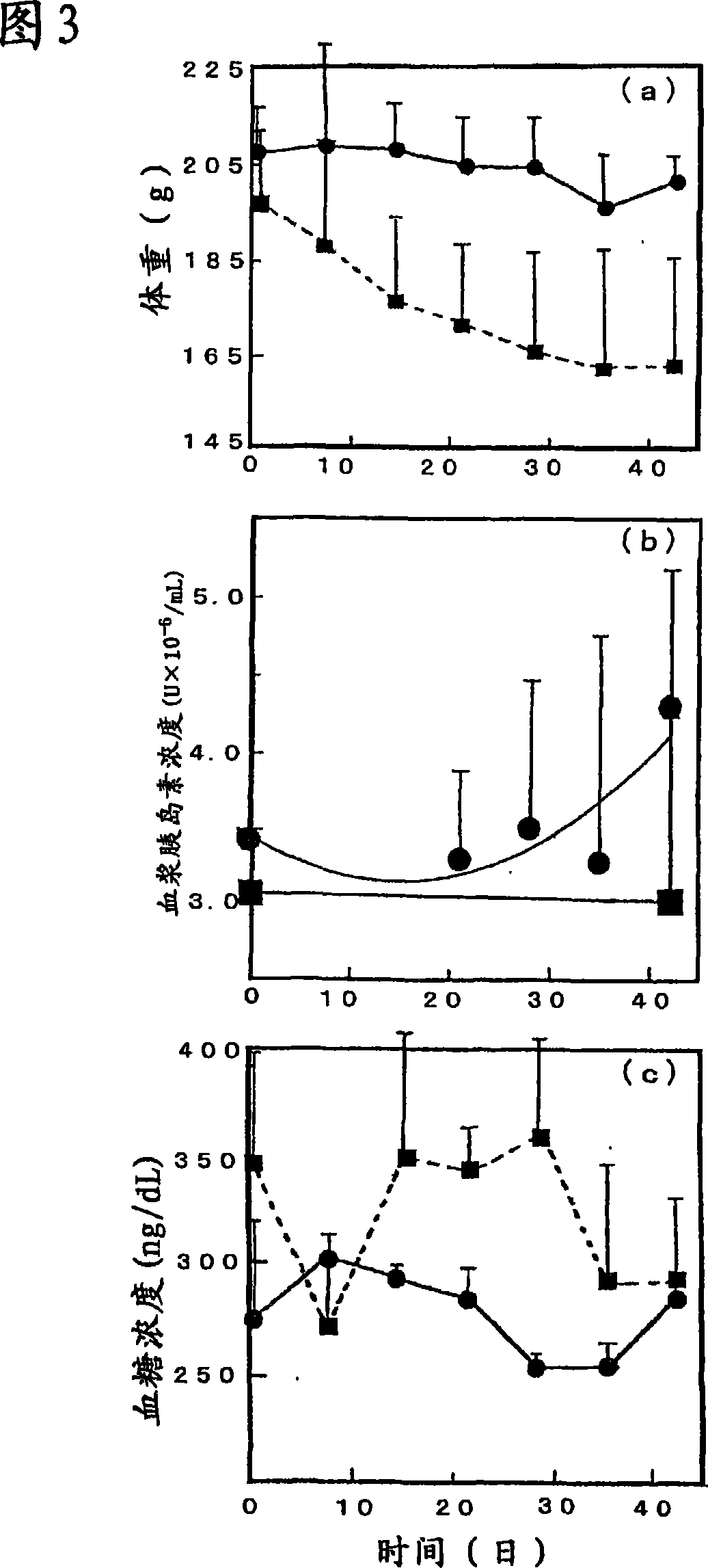
[0062] Body weight changes of diabetic rats administered retinoic acid-organic substance (PLGA) composite particles (6 mg) and control diabetic rats (no retinoic acid administered) up to about 40 days are shown in Figure 3(a). The change of plasma insulin concentration and the change of blood glucose concentration of rats during the period are shown in Figs. 3(b) and 3(c). In the figure, ● represents a rat administered with retinoic acid-organic substance (PLGA) composite particles (6 mg), and ■ represents a control rat (retinoic acid was not administered).
[0063] In control rats not given retinoic acid, a trend was observed, with no change or gradual decrease in plasma insulin concentration, whereas in rats given retinoic acid-organic substance composite particles (LA / PLGA), plasma insulin concentration It was lowest around day 15 and then increased (Fig. 3(b)).
[0064]Further, Fig. 3(c) shows the change of blood glucose concentration, since the data was measured in the c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com