Method and apparatus for purifying inorganic halides and oxyhalides using zeolites
An oxyhalide and halide technology, applied in the preparation method of phosphorus halide/oxyhalide, halide, chemical instruments and methods, etc., can solve the concentration change of inorganic halide and oxyhalide compound, high gas phase purification system. Pressure drop and other problems, to avoid difficult, simple and effective purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0057] The following examples describe the conversion of liquid silicon tetrachloride (SiCl 4 )Remove impurities.
[0058] CBV 400 is a proton form, Y-type zeolite, with 730m 2 / g Brunauer-Emmett-Teller (BET) surface area, SiO 2 / Al 2 o 3 The molar ratio is 5.1 and Na 2 O is 2.8% by weight. The zeolites were reduced in size using a Quaker City Grinding Mill Model F-4 (straub Co., division of Clinton Separators, Inc.; Philadelphia, PA) and classified by sieving to produce zeolites with a particle size greater than 425 microns. The size classified CBV 400 was then dried at about 150°C for about 6 hours under purged nitrogen. Nitrogen was supplied from a large Dewar bottle of liquid nitrogen and purged using a MYDROLIS(R) Inert Gas Purifier model no. WPGV202TI (MYKROLIS(R) is a trademark of Mykrolis Corporation; Billerica MA).
[0059] 10 ml of CBV 400 prepared as described above was placed in a 50 ml buret with a perfluoroalkoxylate (PFA) stopcock. PYREX(R) (trademark of...
Embodiment 1B
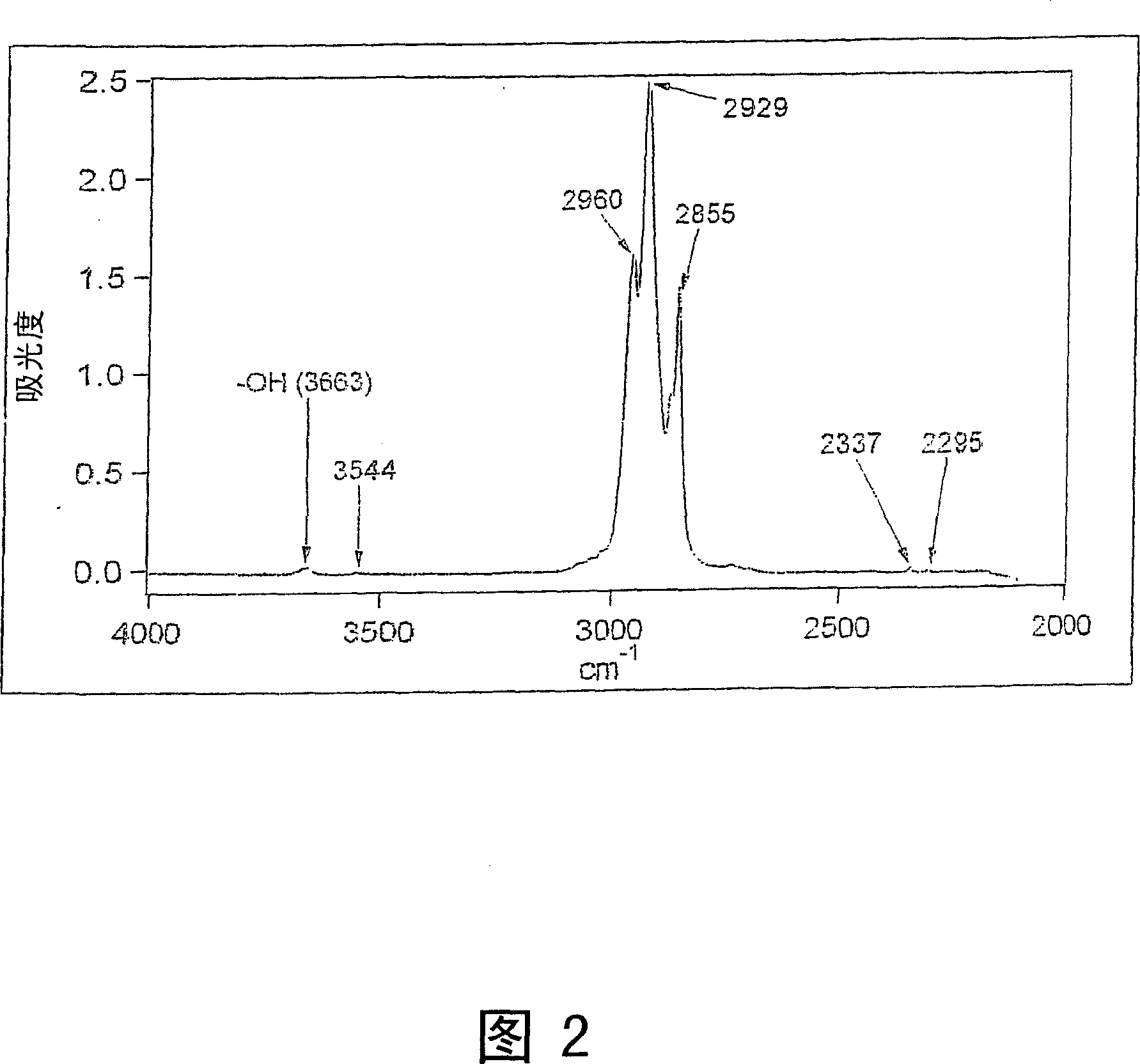
[0063] This example describes the use of Fourier transform infrared (FT-IR) spectroscopic analysis to determine the 4 Efficiency in removing molecular impurities.
[0064] SiCl was measured using a Nicolet Magna 560 FT-IR spectrometer (Thermo Nicolet Corp., Madison, WI) 4 The spectrum of the sample. The background spectrum of an empty 100 ml quartz cell from Wilmad (a division of SP Industries Inc., Buena, NJ) was measured and stored for use as SiCl 4 The background of the sample spectrum. Then, move the quartz cell to the glove box. Take a certain amount of SiCl from the large bottle 4 (99.998%, obtained from Sigma-Aldrich) for flush transfer syringes and quartz cells. Get more SiCl out of the big bottle 4 , filled with a quartz cell and encapsulated. The filled quartz cell was then removed from the glove box, rinsed with high performance liquid chromatography (HPLC) grade isopropanol (IPA), and dried with a Kim-Wipe. Quickly place the quartz cell in the nitrogen-purg...
Embodiment 1C
[0068] This example describes the use of inductively coupled plasma mass spectrometry (ICP-MS) to determine the 4 The efficiency of metal removal.
[0069] Zeolite purified SiCl prepared as described in Example 1A was performed by Chem Trace Inc. (Fremont, CA). 4 and SiCl 4 Analysis of metal content of comparative samples.
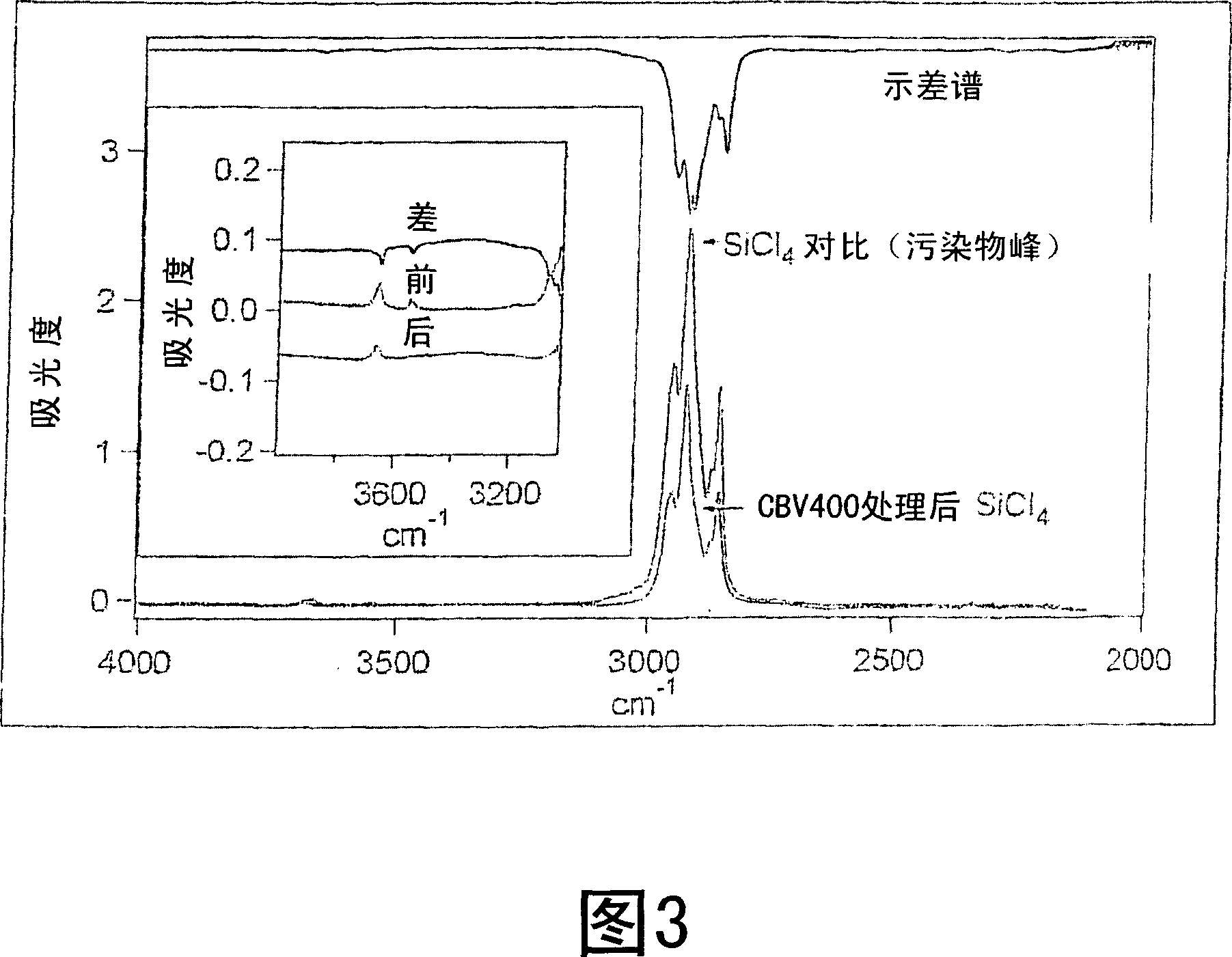
[0070] SiCl 4 The results of the metal analysis of the samples are shown in Table 1. Liquid SiCl purified using CBV 400 was observed 4 Shown as decreasing or unchanged concentrations of all metals except sodium, which changes from 10 per billion (10 9 ) increased from 1.6 parts per billion to 6.6 parts per billion (ppb).
[0071] Metal
[0072] CBV efficiently converts liquid SiCl 4 Removes molecular and metallic impurities. The observed increase in sodium concentration is believed to be due to the relatively high sodium oxide content in the CBV 400 zeolite.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com