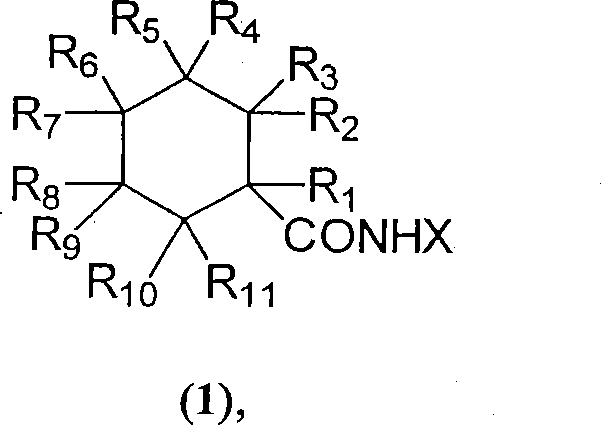
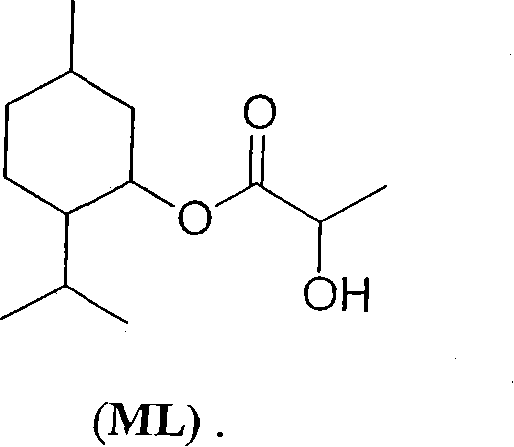
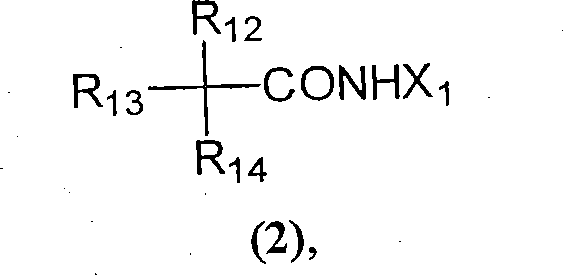Physiological cooling compositions
A composition, physiological technique
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0083] Suitable methods for the preparation of cyclohexanecarboxamides of general formula 1d are shown below and include, but are not limited to, catalyzed cyclization of geranilic acid to cyclogeranonitrile and hydrogenation of cyclogeranonitrile isomers according to the following scheme to dihydrocyclogeranonitrile, which is subsequently reacted with a suitable alkoxy-containing compound, such as an alkanol (X-OH), in the presence of an acid as follows.
[0084]
[0085] Alternatively, the isomeric cyclogeranylnitriles can be first converted to unsaturated cyclogeranylamides followed by hydrogenation to compounds of general formula 1d following the following scheme. The unsaturated cyclogeranylamides shown in the scheme below also have cooling activity and can be used as components of mixtures in place of their saturated analogues.
[0086]
[0087] Preferably at least one cyclohexanecarboxamide or mixture of cyclohexanecarboxamides is incorporated in an amount ranging...
Embodiment 31-38
[0149] Compositions containing WS-3 as cyclohexane carboxamide, WS-23 as acyclic carboxamide and ML containing ML-2S-(1R,2S,5R) stereoisomer
[0150] A mixture of WS-3, WS-23, and menthyl lactate was prepared by co-melting quantitative amounts of WS-3, WS-23, and menthyl lactate and cooling it to ambient laboratory temperature (20-25°C). Mixtures that did not cure spontaneously were mechanically mixed (vibrated) for 0.5-3 minutes and / or seeded with WS-3, WS-23 and / or ML. The results are shown in Table 7 below.
[0151] implement
Embodiment 39
[0153] Compositions containing WS-3 as cyclohexane carboxamide, WS-23 as acyclic carboxamide and ML with ML-2S-(1R,2S,5R) stereoisomer prepared by kneading
[0154] A mixture of 18 g WS-3, 18 g WS-23 and 24 g ML was kneaded in a rotating flask at room temperature and atmospheric pressure for 12 hours. The resulting composition was a clear liquid and contained about 30% WS-3, about 30% WS-23 and about 40% menthyl lactate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com